2014 年 37 巻 1 号 p. 137-144
2014 年 37 巻 1 号 p. 137-144
Tumor-associated macrophages (TAMs) have an alternatively activated macrophage phenotype (M2) and promote cancer cell proliferation, angiogenesis and metastasis. Nuclear factor-kappaB (NF-κB) is one of the master regulators of macrophage polarization. Here, we investigated the effect of inhibition of NF-κB activity by small interfering RNA (siRNA) on the pro-tumor response of macrophages located in the tumor microenvironment in vitro. We used mouse peritoneal macrophages cultured in conditioned medium from colon-26 cancer cells as an in vitro TAM model (M2-like macrophages). Transfection of NF-κB (p50) siRNA into M2-like macrophages resulted in a significant decrease in the secretion of interleukin (IL)-10 (a T helper 2 (Th2) cytokine) and a significant increase of T helper 1 (Th1) cytokine production (IL-12, tumor necrosis factor-α, and IL-6). Furthermore, vascular endothelial growth factor production and matrix metalloproteinase-9 mRNA expression in M2-like macrophages were suppressed by inhibition of NF-κB expression with NF-κB (p50) siRNA. In addition, there was a reduction of arginase mRNA expression and increase in nitric oxide production. The cytokine secretion profiles of macrophages cultured in conditioned medium from either B16BL6 or PAN-02 cancer cells were also converted from M2 to classically activated (M1) macrophages by transfection of NF-κB (p50) siRNA. These results suggest that inhibition of NF-κB activity in M2-like macrophages alters their phenotype toward M1.
Tumor-associated macrophages (TAMs) play a crucial role in tumor progression. TAMs have a phenotype closely resembling the M2 phenotype and show anti-inflammatory and pro-tumor functions.1–3) So far, many clinical studies have revealed that the presence of TAMs are strongly correlated with a poor prognosis for cancer patients.4–6) Macrophages mainly polarize into two phenotypes with different cytokine and growth factor production profiles.7,8) Classically activated macrophages (M1 macrophages) have an interleukin (IL)-10low and IL-12high phenotype.9,10) These macrophages express high levels of major histocompatibility complex molecules and produce large amounts of nitrogen oxide (NO) and inflammatory cytokines such as IL-6, IL-1β, and tumor necrosis factor (TNF)-α. In contrast, alternatively activated macrophages (M2 macrophages) have an IL-10high and IL-12low phenotype and produce several growth factors such as vascular endothelial growth factor (VEGF) and platelet-derived growth factor. In addition, M2 macrophages express a high level of mannose receptor on their surface.9,10) Because TAMs show an M2 phenotype in response to a tumor microenvironment,11,12) regulation of TAM functions is a promising approach for cancer therapy.13,14) In particular, conversion of the phenotype and immune response of TAMs from M2 to M1 is one of the most effective strategies because of not only suppression of M2 pro-tumor functions, but also enhancement of the M1 tumoricidal activity.15–17)
Recent studies have shown that a number of signaling pathways directly affect the polarization and function of macrophages.18–20) Notably, nuclear factor-kappaB (NF-κB) is an important transcription factor that regulates macrophage inflammatory and immune responses, and is considered as a master regulator of macrophage phenotypes.21,22) Hagemann et al. have reported that TAMs isolated from an NF-κB activation-knockout mouse show an M1 phenotype.15) These studies led us to believe that activation of inhibitor of NF-κB kinase (IKK)β/NF-κB may be required to maintain the M2 phenotype of TAMs. Therefore, we investigated whether inhibition of NF-κB activity in TAMs can suppress their pro-tumor functions through conversion of their phenotype from M2 to M1.
In this study, we examined the effects of inhibition of NF-κB activity with NF-κB (p50) small interfering RNA (siRNA) on the phenotype of primary cultured M2-like macrophages. Because the phenotype of macrophages is classified by the above-mentioned factors, we focused on cytokine and growth factor production, NO production, and the receptor expression characteristics of M1 and M2 phenotypes. Cultivated mouse peritoneal macrophages in conditioned medium from cancer cells were used as an in vitro experimental model of TAMs.
All animal experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals by the U.S. National Institutes of Health (Bethesda, MD, U.S.A.) and the Guidelines for Animal Experiments of Kyoto University (Kyoto, Japan). The protocol was approved by the Kyoto University Animal Experimentation Committee (approval number 2012-32). All surgery was performed under sodium pentobarbital anesthesia.
Macrophages and Cell LinesMouse peritoneal elicited macrophages (PEM) were collected from 4–5-week-old female ICR mice injected 4 d previously with 1 mL of 2.9% thioglycolate medium (Nissui, Tokyo, Japan) and cultured according to our previously published methods.23) PEM were seeded in 24-well culture plates at a density of 5×105 cells/1.88 cm2. After incubation for 2 h at 37°C with 5% CO2, non-adherent cells were washed off with culture medium. Colon-26 mouse colon adenocarcinoma cells and B16BL6 mouse melanoma cells were obtained from the Riken Bioresource Center (Osaka, Japan). PAN-02 mouse pancreatic ductal adenocarcinoma cells were purchased from the Division of Cancer Treatment and Diagnosis, National Cancer Institute (Frederick, MD, U.S.A.). All cancer cells were cultured in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum, penicillin G (100 U/mL), and streptomycin (100 µg/mL) at 37°C with 5% CO2.
NF-κB (p50) siRNAsiRNA targeting NF-κB p50 (NF-κB (p50) siRNA) and scrambled siRNA were purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.). The sequences of the siRNAs were as follows: NF-κB siRNA: sense, CAC AUC UGA UGA UAU ACU ATT; antisense, UAG UAU AUC AUC AGA UGU GTT scrambled siRNA: sense, AAG UAA ACG CAC GUU UAC CTT; antisense, GGU AAA CGU GCG UUU ACU UTT
Preparation of M2-Like MacrophagesTo obtain conditioned medium from cancer cells, the cells were seeded at 5×105 cells/78.5 cm2 and cultivated to 80% confluence. The supernatants were harvested and filtered through a 0.45 µm membrane filter (Nihon-Millipore, Tokyo, Japan). The obtained conditioned medium contained no detectable IL-10, IL-12p70, TNF-α and IL-6, as confirmed by the commercial ELISA kits (Bay Bioscience Co., Ltd., Hyogo, Japan). M2-like macrophages were prepared by culturing PEM in conditioned medium from cancer cells. At 24 h prior to transfection of siRNA into macrophages, the medium was changed to conditioned medium from cancer cells.
In Vitro Transfection of NF-κB (p50) siRNA into M2-Like MacrophagesAfter PEM were cultured in conditioned medium from cancer cells for 24 h, the medium was replaced with Opti-MEM-I (Gibco BRL, Grand Island, NY, U.S.A.), and siRNA was added with Lipofectamine® 2000 (Invitrogen, Carlsbad, CA, U.S.A.). After 6 h of incubation, the medium was replaced with conditioned medium, and the cells were incubated for an additional 18 h.
Quantification of mRNA in MacrophagesAt 24 h later after siRNA transfection, total RNA was isolated from PEM using a GenElute Mammalian Total RNA Miniprep Kit (Sigma-Aldrich). Reverse transcription of messenger RNA (mRNA) was carried out using a PrimeScript® RT Reagent Kit (TaKaRa Bio Inc., Shiga, Japan). The detection of cDNA (p50, p65, matrix metalloproteinase-9 (MMP-9), arginase, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH)) was conducted using real-time polymerase chain reaction (PCR) with SYBR® Premix Ex Taq (TaKaRa Bio Inc.) and a Lightcycler Quick System 350S (Roche Diagnostics, Indianapolis, IN, U.S.A.). The primers for p50, p65, MMP-9, arginase and GAPDH were as follows: p50, 5′-CCT GGA TGA CTC TTG GGA AA-3′ (forward) and 5′-TCA GCC AGC TGT TTC ATG TC-3′ (reverse); p65, 5′-TAG CAC CTG ATG GCT GAC TG-3′ (forward) and 5′-CGT TCC ACC ACA TCT GTG TC-3′ (reverse); MMP-9, 5′-GAA GGC AAA CCC TGT GTG TT-3′ (forward) and 5′-AGA GTA CTG CTT GCC CAG GA-3′ (reverse); arginase, 5′-GTG AAG AAC CCA CGG TCT GT-3′ (forward) and 5′-CTG GTT GTC AGG GGA GTG TT-3′ (reverse); GAPDH, 5′-TCT CCT GCG ACT TCA ACA-3′ (forward) and 5′-GCT GTA GCC GTA TTC ATT GT-3′ (reverse).
Western Blottingwestern blotting was performed according to our previous published methods.24) Macrophages were collected at 24 h after siRNA transfection into macrophages, and nuclear extracts from macrophages were prepared using a Nuclear Extract Kit (Active Motif, Carlsbad, CA, U.S.A.). To detect p50, p65, and β-actin, the following antibodies were used: rabbit anti-mouse p105/p50 (1 : 400), rabbit anti-mouse p65 (1 : 400) (Abcam, Cambridge, U.K.), and rabbit anti-mouse β-actin (1 : 2000) (Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.). A horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG) (1 : 2000) (Santa Cruz Biotechnology) was used as the secondary antibody. Antibodies were diluted in Tris-buffered saline containing 1% bovine serum albumin. Membranes were stripped before reactions with subsequent primary antibodies.
Measurement of Cytokine and VEGF Production from MacrophagesAt 24 h after the culture medium was changed to conditioned medium, lipopolysaccharide (LPS) (100 ng/mL; Sigma-Aldrich) was added to the culture medium, and the culture supernatants were collected at various times. At 24 h after siRNA transfection into macrophages, LPS (100 ng/mL) was added to the culture medium, and the culture supernatants were collected at 24 h after LPS stimulation. The concentrations of IL-10, IL-12p70, TNF-α and IL-6 in culture supernatants were measured with commercial ELISA kits (Bay Bioscience Co., Ltd.). The VEGF-A concentration in culture supernatants was also measured with a commercial ELISA kit (PeproTech Inc., Rocky Hill, NJ, U.S.A.).
Griess AssayAt 24 h after LPS stimulation of macrophages, the culture supernatants were collected. NO production from macrophages was measured by the Griess assay using a Griess Reagent System (Promega, Madison, WI, U.S.A.).
Flow CytometryAt 24 h after siRNA transfection into macrophages, the macrophages were washed, resuspended in PBS containing 5% fetal bovine serum, and stained with a fluorescein isothiocyanate (FITC)-labeled rat anti-mouse CD206 antibody (Serotec, Oxford, U.K.) or FITC-labeled rat anti-mouse CD204 antibody (AbD Serotec, Raleigh, NC, U.S.A.) at a final concentration of 10 µg/mL for 30 min on ice. After washing, the labeled cells were analyzed by a FACSCanto™ II (Becton, Dickinson and Company, Tokyo, Japan).
Statistical AnalysisResults are presented as the means±or+standard deviation (S.D.) of four experiments. ANOVA was used to test the statistical significance of differences between groups. Two-group comparisons were performed with the Student’s t-test. Multiple comparisons between control groups and other groups were performed with the Dunnett’s test, and multiple comparisons between all groups were performed with the Tukey–Kramer test.
T helper 2 (Th2) cytokine (IL-10) production by PEM cultured in conditioned medium from colon-26 cells was much higher than that by PEM cultured in unconditioned RPMI-1640 medium (Fig. 1A). In addition, PEM cultured in conditioned medium from colon-26 cells secreted low levels of T helper 1 (Th1) cytokines (IL-12, TNF-α, and IL-6) (Figs. 1B–D). Therefore, we used these M2-like macrophages as an in vitro TAM model.
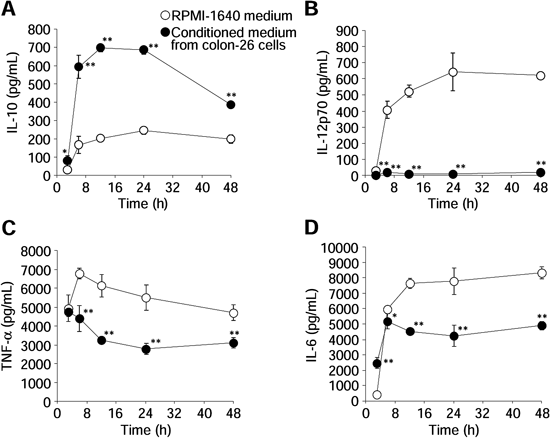
Time course of the concentrations of IL-10 (A), IL-12p70 (B), TNF-α (C), and IL-6 (D) in culture supernatants of macrophages after LPS stimulation (100 ng/mL). Data represent the means±S.D. (n=4). * p<0.05, ** p<0.01 compared with PEM cultured in RPMI-1640 medium.
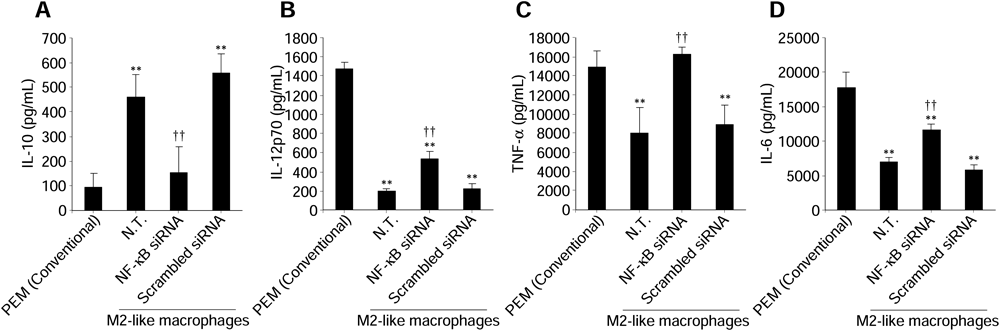
We evaluated the effects of inhibiting NF-κB expression by NF-κB (p50) siRNA on cytokine production from M2-like macrophages. As shown in Figs. 2A, B, NF-κB (p50) siRNA specifically cleaved p50 mRNA and inhibited subsequent p50 protein production in M2-like macrophages. In addition, intranuclear p65 was slightly decreased following the reduction of intranuclear p50. IL-10 secretion was significantly reduced from M2-like macrophages (Fig. 2C). Moreover, the secretion of IL-12, TNF-α, and IL-6 was significantly increased from M2-like macrophages (Figs. 2D–F).
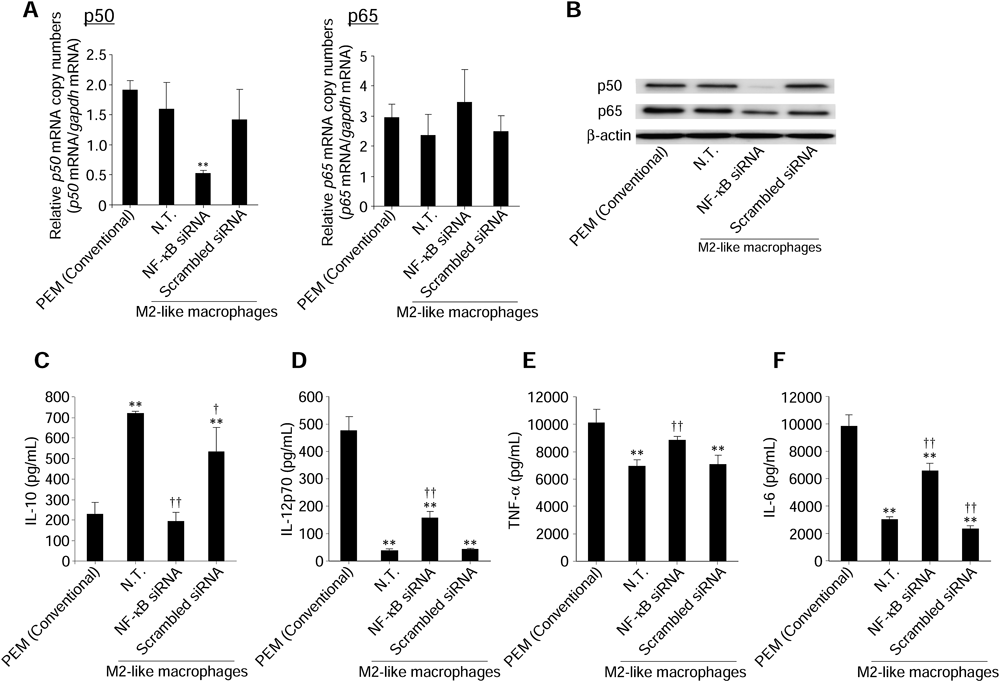
Expression levels of p50 and p65 mRNA (A) and intranuclear p50 and p65 levels (B) in macrophages were measured at 24 h after transfection of NF-κB (p50) siRNA (0.1 µM). The concentrations of IL-10 (C), IL-12p70 (D), TNF-α (E), and IL-6 (F) in culture supernatants were measured following transfection of NF-κB (p50) siRNA (0.1 µM) at 24 h after LPS stimulation. Data represent the means+S.D. (n=4). ** p<0.01 compared with PEM (Conventional). † p<0.05, †† p<0.01 compared with N.T. of M2-like macrophages. N.T.: Non-treated cells.
VEGF and MMP-9 are preferentially expressed by M2 macrophages and mainly contribute to angiogenesis and metastasis.25,26) After transfection of NF-κB (p50) siRNA, we evaluated VEGF and MMP-9 expression levels in M2-like macrophages. Both VEGF production and MMP-9 mRNA expression were significantly decreased in M2-like macrophages (Fig. 3).

(A) VEGF concentrations in culture supernatants were measured following transfection of NF-κB (p50) siRNA (0.1 µM) at 24 h after LPS stimulation. (B) The expression level of MMP-9 mRNA in macrophages was measured at 24 h after transfection of NF-κB (p50) siRNA (0.1 µM). Data represent the means+S.D. (n=4). ** p<0.01 compared with PEM (Conventional). † p<0.05, †† p<0.01 compared with N.T. of M2-like macrophages. N.T: Non-treated cells.
Macrophages use L-arginine to synthesize NO through inducible NO synthase (iNOS) and to produce L-ornithine through arginase.27,28) M1 macrophages express low levels of arginase and high levels of iNOS for efficient NO production, whereas M2 macrophages exhibit the opposing trend.27,28)
As shown in Fig. 4A, we observed significant suppression of arginase gene expression in M2-like macrophages by transfection of NF-κB (p50) siRNA. Moreover, NO2− production from M2-like macrophages was significantly increased by transfection of NF-κB (p50) siRNA (Fig. 4B).
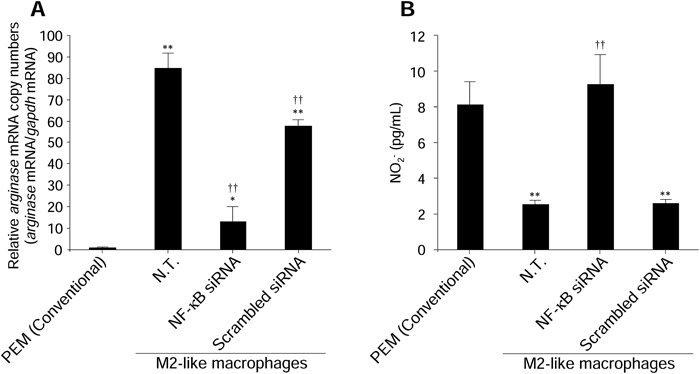
(A) The expression level of arginase mRNA in macrophages was measured at 24 h after transfection of NF-κB (p50) siRNA (0.1 µM). (B) NO2− concentrations in culture supernatants were measured following transfection of NF-κB (p50) siRNA (0.1 µM) at 24 h after LPS stimulation. Data represent the means+S.D. (n=4). * p<0.05, ** p<0.01 compared with PEM (Conventional). †† p<0.01 compared with N.T. of M2-like macrophages. N.T.: Non-treated cells.
Mannose receptor and scavenger receptor are one of the major proteins expressed on the surface of M2 macrophages.11,29) Transfection of NF-κB (p50) siRNA into M2-like macrophages resulted in a significantly reduction of both of these receptors expression (Fig. 5).
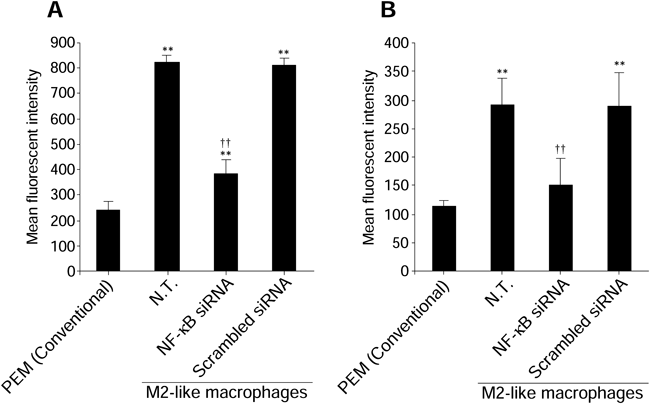
The expression levels of mannose receptor (A) and scavenger receptor (B) on the surface of macrophages were measured at 24 h after transfection of NF-κB (p50) siRNA (0.1 µM). Data represent the means+S.D. (n=4). ** p<0.01 compared with PEM (Conventional). †† p<0.01 compared with N.T. of M2-like macrophages. N.T.: Non-treated cells.
Because macrophages have functional plasticity and can change their phenotype in response to their microenvironment, we prepared two other kinds of M2-like macrophages using conditioned medium from B16BL6 mouse melanoma cells and PAN-02 mouse pancreatic ductal adenocarcinoma cells. We then evaluated the effects of inhibiting NF-κB activity using NF-κB (p50) siRNA on cytokine secretion from these M2-like macrophages.
We observed a significant reduction of IL-10 secretion by inhibition of NF-κB activity using NF-κB (p50) siRNA in M2-like macrophages treated with conditioned medium from either B16BL6 or PAN-02 cells (Figs. 6A, 7A). In addition, we observed a significant increase in IL-12, TNF-α, and IL-6 production (Figs. 6B–D, 7B–D).

Concentrations of IL-10 (A), IL-12p70 (B), TNF-α (C), and IL-6 (D) in culture supernatants were measured following transfection of NF-κB (p50) siRNA (0.1 µM) at 24 h after LPS stimulation. Data represent the means+S.D. (n=4). * p<0.05, ** p<0.01 compared with PEM (Conventional). †† p<0.01 compared with N.T. of M2-like macrophages. N.T.: Non-treated cells.
Concentrations of IL-10 (A), IL-12p70 (B), TNF-α (C), and IL-6 (D) in culture supernatants were measured following transfection of NF-κB (p50) siRNA (0.1 µM) at 24 h after LPS stimulation. Data represent the means+S.D. (n=4). ** p<0.01 compared with PEM (Conventional). †† p<0.01 compared with N.T. of M2-like macrophages. N.T.: Non-treated cells.
TAMs closely resemble alternatively activated M2 macrophages that exhibit an IL-10high and IL-12low phenotype.1–3,9,10) In contrast, classically activated M1 macrophages have an IL-10low and IL-12high phenotype and produce NO and inflammatory cytokines such as TNF-α and IL-6.1–3,9,10) Therefore, we evaluated the production levels or gene expression of these factors as indications of the phenotype of macrophages.
Because the numbers of TAMs in mouse tumor tissues are limited, it is difficult to use TAMs isolated from mouse tumors for in vitro transfection experiments. In addition, it is difficult to maintain the M2 phenotype of TAMs after isolation from mouse tumor tissue because of the plasticity of macrophage phenotypes.30) Alternatively, some researchers have reported that co-cultivation of macrophages with cancer cells changes the cytokine and MMP production properties of macrophages.31–33) Therefore, we prepared M2-like macrophages by culturing PEM in conditioned medium from colon-26 cancer cells. We confirmed that these M2-like macrophages produced a large amount of a Th2 cytokine (IL-10) (Fig. 1A) and low levels of Th1 cytokines (IL-12, TNF-α, and IL-6) (Figs. 1B–D). Similar results were observed when PEM were cultured in conditioned medium from B16BL6 (Fig. 6) or PAN-02 (Fig. 7) cancer cells. The cytokine secretion profiles of these M2-like macrophages are in accordance with those of macrophages co-cultured with cancer cells15,34) or TAMs30) in previous studies. In one experiment, we used LPS as a stimulator of cytokines production from macrophages. LPS is reported to have the potential to direct polarization of monocytes/macrophages towards the M1 phenotype.11,12) On the other hand, previous reports have demonstrated that macrophages differentiated to the M2 phenotype exhibit similar cytokines production profiles as those of M2 macrophages, even in the presence of LPS stimulation.34,35) These reports partly support the validity of this experimental model.
Hagemann et al. demonstrated that macrophages with inhibition of NF-κB signaling do not change their phenotype, even in a tumor microenvironment.15) Therefore, the present study was carried out to elucidate the possibility of converting the phenotype of TAMs from M2 to M1 through inhibition of NF-κB activity by transfection of NF-κB (p50) siRNA. NF-κB (p50) siRNA cleaves p50 mRNA through the RNA interference pathway.36) We found that inhibition of NF-κB expression by NF-κB (p50) siRNA converted the characteristics of the M2-like macrophages, including cytokine production (Fig. 2), growth factor and enzyme expression (Figs. 3, 4), and surface receptors expression (Fig. 5), from M2 phenotype to M1 phenotype. To support the findings using conditioned medium from colon-26 cells (Figs. 2–5), we also examined the changes in cytokine production caused by inhibition of NF-κB activity in M2-like macrophages grown in conditioned medium from B16BL6 (Fig. 6) or PAN-02 (Fig. 7) cancer cells. We found that these cells also changed their profile to a Th1 response. These results suggest that inhibition of NF-κB activity has the potential to switch the phenotype of macrophages from M2 to M1.
Although the mechanisms of the relationship between NF-κB activity and macrophage polarization are poorly understood, recent studies have shown the importance of NF-κB signaling for maintenance of the TAM phenotype.21,22) Hagemann et al. reported that down-regulation of NF-κB activation by depletion of IKKβ causes induction of pro-inflammatory gene expression through activation of STAT1 signaling.15) STAT1 is one of the key factors in the M1 polarization of macrophages. Additionally, Porta et al. found that p50 plays a pivotal role in tuning the NF-κB-dependent M1 polarization of TAMs.32,37) They reported that the accumulation of p50 in nucleus was observed in TAM, and these p50 suppressed STAT1 activity, resulted in the suppression of M1 polarization. These reports partly support our findings showing that inhibition of NF-κB activity by NF-κB (p50) siRNA regulates the production of pro-tumor factors and enhances Th1 cytokine secretion from M2-like macrophages.
In conclusion, these results suggest that inhibition of NF-κB activity in M2-like macrophages using NF-κB (p50) siRNA suppresses the expression of pro-tumor factors and enhances the production of tumoricidal factors. In this study, PEM cultured in conditioned medium from colon-26 cancer cells as an in vitro TAM model (M2-like macrophages) were used for the evaluation; thus in vivo effects remain to be clarified. To evaluate the possibility for its in vivo application, we are ongoing to develop a TAM-selective delivery system in tumor-bearing mice.
This work was supported in part by a Grant-in-Aid for Scientific Research on Innovative Areas from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and by the Programs for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation (NIBIO).