2014 年 37 巻 12 号 p. 1982-1985
2014 年 37 巻 12 号 p. 1982-1985
The bursa of Fabricius (BF) is a unique primary lymphoid organ, and among vertebrates is unique to birds. Despite its importance to the immune systems of various avian species, little is known of the molecular mechanisms underlying early BF development. In the present study, we demonstrated that apoptosis occurs during early development of the bursa of Fabricius in chicken embryos. Initial histological analyses of BF morphogenesis in chicken embryos led to the hypothesis that formation of the bursal lumen correlates with fusion of vacuoles, which appear in the cloacal epithelial bud. Using terminal deoxynucleotidyl transferase deoxyuridine triphosphate (dUTP) nick-end labeling (TUNEL) analysis and immunostaining with an anti-cleaved (activated) caspase-3 antibody, we detected multiple apoptotic cells around these vacuoles. In further experiments, treatments with a caspase inhibitor caused abnormal bursal lumen in vivo. The present data indicate that apoptosis may play important roles in BF morphogenesis in chickens.
Studies of species other than mice and humans have contributed greatly to the understanding of immunology. Among these species, birds have provided an important basis for investigating immunological mechanisms. The bursa of Fabricius (BF) is a unique primary lymphoid organ that is observed only in birds, and is responsible for the development and differentiation of B lymphocytes.1–3) In adult birds, the BF is located between the cloaca and the sacrum and is connected to the proctodeum, which is the caudal portion of the cloaca. The anlage of the BF arises as an epithelial bud in the cloaca in E4.5–E5 chicken embryos.4,5) Between E11 and E15, hematopoietic cells enter the growing bursal mesenchyme, and surface epithelium and bursal follicles are formed.6) Although the origin of the bursal primordium has been controversial for over a century,4,5,7–9) the ectodermal origin of the bursal anlage has been recently demonstrated using chick-quail chimera and tissue recombination methods.10)
Despite the importance of the BF to immune systems of avian species, the molecular mechanisms underlying early BF development are not as well characterized as those involved in thymus development.11) Hence, the aim of this study was to investigate developmental processes of the bursal lumen, bursal duct, and proctodeum in chicken embryos, and to examine the molecular mechanisms behind these processes. Detailed histological analyses of chicken embryos were initially performed to examine BF morphogenesis, and in agreement with previous reports, multiple vacuoles were observed in developing cloacal epithelium. In terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate (dUTP) nick-end labeling (TUNEL) experiments and immunostaining analyses with an anti-cleaved (activated) caspase-3 antibody, numerous apoptotic cells were detected around these vacuoles, suggesting a close relationship with apoptosis and the appearance of the vacuoles. In addition, the caspase inhibitor acetyl-Asp-Glu-Val-Asp (AcDEVD)-cmk induced unusual development of BF in vivo, indicating an important role of apoptosis in morphogenesis during early development of the BF in chicken.
Fertilized chicken eggs were purchased from a local supplier and were incubated at 37°C. Embryos were staged according to Hamburger and Hamilton,12) and were fixed using 10% (v/v) neutral buffered formalin or Serra’s fixative. For histological, TUNEL, and immunohistochemical analyses, embryos were embedded in paraffin wax after fixation and were then sectioned (8 µm).
Histological Examination, TUNEL Assay, and ImmunohistochemistryDeparaffinized sections were stained with hematoxylin and eosin (H&E) for histological examinations. TUNEL assays were performed to detect DNA fragmentation that is characteristic of apoptotic nuclei using an ApopTag Plus Peroxidase in Situ Apoptosis Detection Kit (cat # S7101, Merck Millipore, MA, U.S.A.) according to the manufacturer’s protocols. For immunohistochemical analyses, deparaffinized and rehydrated sections were pretreated in a microwave oven (600 W) for 30 min in 10-mM citrate buffer (pH 6.0). Slides were then rinsed in phosphate buffered saline (PBS) and were incubated overnight at 4°C in PBS containing anti-cleaved (activated) caspase-3 antibody (cat # C8487, Sigma-Aldrich, MO, U.S.A.) at a dilution of 1 : 200. Alexa Fluor 488-conjugated goat anti-rabbit IgG (Molecular Probes, Eugene, OR, U.S.A.) was subsequently used to detect background fluorescence staining. Slides were mounted using ImmunoSelect Antifading Mounting Medium DAPI (cat # SCR-38448, Dianova GmbH, Hamburg, Germany).
In Vivo Administration of the Caspase Inhibitor AcDEVD-cmkAcDEVD-cmk (Merck, Millipore, MA, U.S.A.) is an irreversible, competitive inhibitor of caspases 3, 6, 7, 8, and 10, and was administered as described by Milligan et al.13) Briefly, stock solutions of AcDEVD-cmk in dimethyl sulfoxide (DMSO) were diluted to appropriate concentrations (0.001–10 mM) in PBS, and 100-µL solutions were dropped onto highly vascularized chorioallantoic membranes through windows in the shell. An equivalent volume of DMSO/PBS was administered to control embryos. Embryos were given a single dose of the agent E6, and were sacrificed 24 h later and fixed as described above.
Although early development of the BF in chicken embryos has been described previously,4,5) we systematically examined serial histological sections of stage (st.) 26–32 chicken embryos, and elucidated the developmental processes of the BF in greater detail. During the first step of BF formation in st. 26 chicken embryos, epithelial buds develop from the caudal portion of the hindgut (urodeum; Fig. 1A). Accordingly, we observed growth and invasion of endodermal epithelial buds into tail bud mesenchymal cells in st. 28 embryos (Fig. 1B), and small vacuoles were observed in growing epithelial buds (Fig. 1C, arrowhead). Concomitant with bud growth, we observed anal invagination toward the dorsal region (Fig. 1B, black arrow), and the numbers and sizes of vacuoles increased with endodermal epithelial bud growth. Numbers of vacuoles were further increased in st. 30 embryos, and the bursal lumen appeared to form with coalescence of vacuoles (Fig. 1D). In st. 31 embryos, the bursal lumen was connected to the proctodeum, but was separate from the urodeum (Fig. 1E). Finally, in st. 32 embryos, the bursal duct was established between the bursal lumen and the cloacal membrane (Fig. 1F).
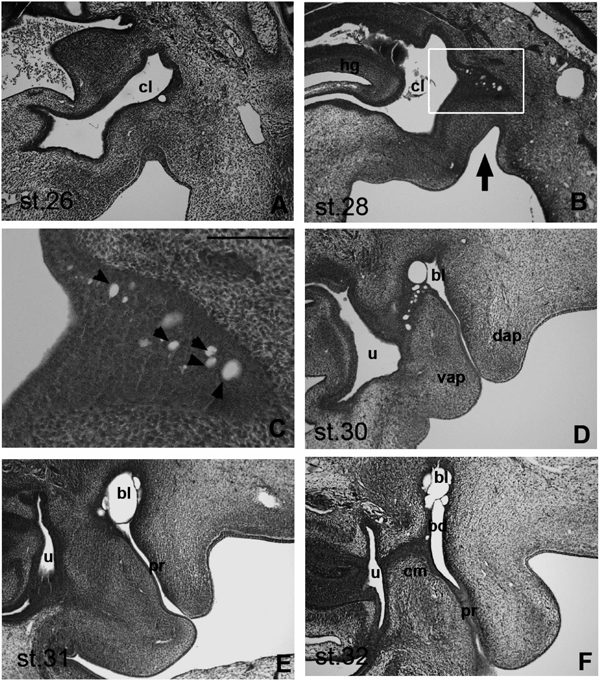
Sagittal H&E section of the tail region in st. 26 (A), 28 (B), 30 (D), 31 (E), and 32 (F) chicken embryos. The boxed area in B is magnified in C. In B, the black arrow indicates anal invagination. In C, arrowheads indicate vacuoles; bd, bursal duct; bl, bursal lumen; cl, cloaca; cm, cloacal membrane; dap, dorsal anal lip; hg, hind gut; pt, proctodeum; u, urodeum; vap, ventral anal lip. Scale bars in B and C, 100 µm.
These observations suggest that the bursal lumen is derived from the fusion of vacuoles in the endoderm and the invaginated ectoderm. However, experimental data using chick-quail chimera and tissue recombination methods suggest that the ectoderm, rather than the endoderm, is the origin of bursal epithelial primordium.10) Hence, to assess the discrepancy between our morphological analyses, which raised the possibility of participation of endoderm and ectoderm in the formation of the bursal lumen, and the chimera experiment,10) we investigated mechanisms of vacuolation in the cloacal epithelial bud. Specifically, we assessed the involvement of endoderm vacuoles in the development of bursal epithelial primordium, and determined whether inhibition of vacuole formation results in defective or incomplete BF.
Interestingly, a similar phenomenon was observed during cloacal development in rats, which are placental mammals.14) In this study, a number of small vacuoles were reported in the future urethral opening, and coalescence of these resulted in communication between urethral and amniotic cavities. Multiple apoptotic cells were detected in this region prior to vacuole formation, indicating that vacuolation may be a consequence of apoptosis.14) Accordingly, we hypothesized that the vacuoles observed in epithelial buds during chicken BF development were also derived from apoptotic processes.
Distribution of Apoptosis during BF DevelopmentTo analyze the distribution of apoptotic cells during the development of BF, we performed TUNEL staining and immunostaining analyses using an antibody against the cleaved (activated) caspase 3. TUNEL staining demonstrated the appearance of apoptotic cells in the epithelial bud, the caudal cloacal epithelium, and in mesenchymal cells, which partition the dorsal region of the bud in st. 28 and 30 embryos (Figs. 2B, C, E, F). Moreover, apoptotic cells were distributed uniformly in the epithelial bud (Figs. 1C, F). TUNEL staining detects both non-apoptotic and apoptotic cell death. Thus, we confirmed the role of apoptosis in BF development using immunostaining analyses with an antibody against activated caspase 3 in st. 28 chicken embryos (Figs. 2G, H). Distribution patterns of activated caspase 3-expressing cells in developing BF were similar to those of positively stained cells in TUNEL assays. Taken together, these data indicate that apoptosis occurs in cloacal epithelial buds in chicken embryos, and that vacuolation in epithelial buds may reflect apoptosis.
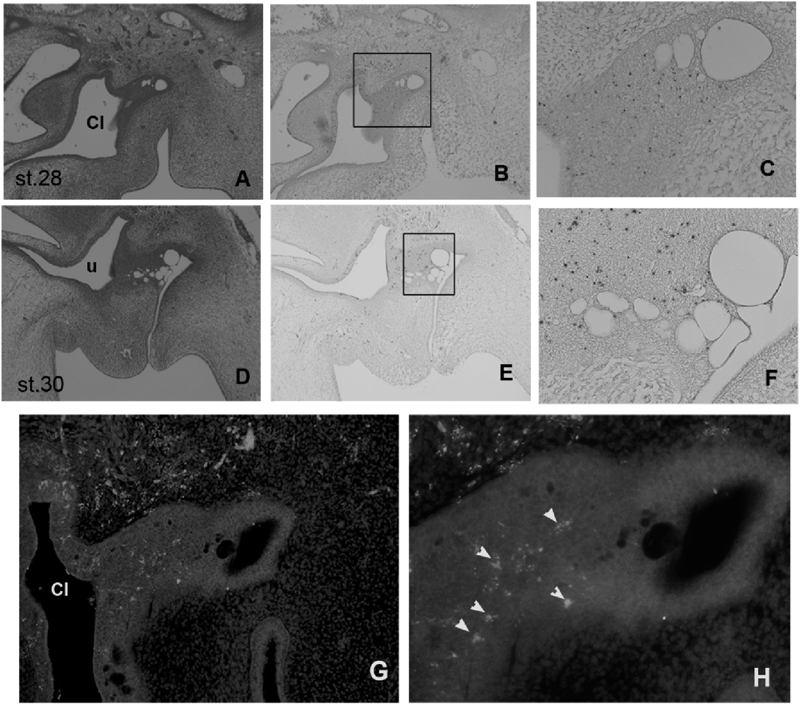
(A–F) Sagittal sections of the tail region in st. 28 (upper) and st. 30 (lower) chicken embryos were stained with H&E (A, D) or with TUNEL (B, C, E, F); C and F are high-magnification pictures of B and E. In (C) and (F), nuclei displaying DNA fragmentation are stained in black. (G) Sagittal section of the tail region in st. 28 chicken embryos after immunostaining with an anti-activated caspase 3 antibody; (H) High-magnification picture of G; Arrowheads indicate activated caspase 3-expressing cells; cl, cloaca; u, urodeum.
To examine the role of apoptosis in the development of BF, we administrated the caspase inhibitor AcDEVD-cmk to chicken embryos in vivo. Subsequent histological analyses showed that treatments with the inhibitor at 0.001–5 mM had no effect on bursal development in chicken embryos (data not shown). However, interesting morphological changes were observed in embryos treated with the inhibitor at 10-mM (2 embryos/4 treated embryos; Fig. 3B). In comparison with the bursal lumen shown in Fig. 1D and Fig. 3A, the developing bursal lumen in Fig. 3B appears to be abnormal. Moreover, ectopic mesodermal cell aggregation was observed in the proctodeum (Fig. 3B, arrow), and numbers of vacuoles appeared to be fewer. These results suggest that apoptosis in the developing BF may important roles in morphogenesis during bursal development. However, further experiments are required to confirm anti-apoptotic mechanisms of caspase inhibitors during BF development.
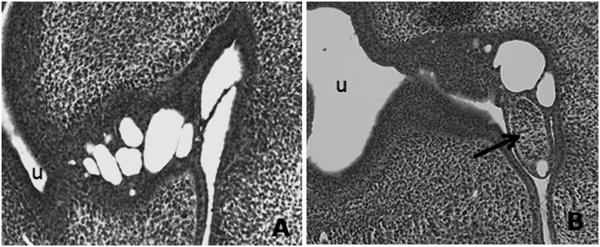
Sagittal section of a H&E stained embryos after treatment with DMSO/PBS (A) or 10-mM AcDEVD-cmk (B); The arrow in B indicates aggregation of mesodermal cells; u, urodeum.
It is accepted that apoptosis plays important roles in animal development.15–19) Pivotally, Miller and Briglin suggested that apoptosis is central to removal of the tail gut, and is linked to eventual formation of the BF in chicken embryos.9) Moreover, apoptosis plays a role in urethral opening during cloacal development in mammals,20) and in opening of the vagina, which is a cloacal derivative.21) Potentially, formation of bursal lumen is regulated in a manner similar to that of cloacal derivatives in placental mammals. In mice, bone morphogenetic protein 7 (Bmp7) signaling controls partitioning in the cloacal endoderm, resulting in topological separation of urinary and digestive systems,22) and is known to promote cell survival in the cloacal endoderm. Accordingly, we speculate that Bmp signaling may play a role in BF development.
In this study, we demonstrated that apoptosis occurs during early development of the BF in chicken embryos, and may play important roles in BF morphogenesis. Further studies are required to fully understand the roles of apoptosis in BF development.