2014 年 37 巻 6 号 p. 1050-1055
2014 年 37 巻 6 号 p. 1050-1055
Dried Nardostachys chinensis roots contain sesquiterpenoids that are widely used as herbal tranquilizers. We previously identified the highly sedative sesquiterpenoid valerena-4,7(11)-diene (VLD) from this plant. In the present study, we investigated stress reducing effects of VLD and the associated mechanisms of action. Application of 15-min restraint stresses induced excitatory behaviors in mice. Immobility times in the forced swim test and sleeping times in the pentobarbital sleep test were shortened in the stressed group by 47% and 43%, respectively, compared with the control group. Furthermore, restraint stress increased serum corticosterone levels by 75%, and cerebral serotonin (5-HT) and dopamine (DA) levels. Inhaled VLD (300 µg/cage) suppressed stress-induced excitatory behaviors and significantly reduced stress-induced blood corticosterone, cerebral 5-HT, and DA levels. These results suggest that VLD interacts with the hypothalamic–pituitary–adrenal axis and the sympathetic-adrenomedullary system. These interactions appear to involve GABAergic and D2 antagonist activities. Moreover, tests in anosmic and intravenously treated mice showed that the sedative effect of inhaled VLD was expressed via olfactory stimulation and pulmonary absorption. Although more studies are required to further elucidate the properties of this compound, our studies suggest that VLD may be an effective anti-stress aromatherapy for humans.
Various daily stress significantly influence health and may evoke physical disorders such as insomnia and dyspepsia.1) Anxiolytic or psycholeptic agents are widely used as sedative drugs, but they are associated with problematic side effects such as drug dependence.2) Therefore, as one of the complementary and alternative medicines, aromatherapy has recently attracted significant attention for its ability to ameliorate stress. In our previous study, we identified natural tranquilizing vapors from spikenard extracts, and showed marked sedative effects after inhalation in mice.3) Spikenard, the dried root of Nardostachys chinensis Batal. (Valerianaceae), which is abundant in sesquiterpenoids, has a long history as an essential ingredient in scented sachets and has been used as a traditional herbal tranquilizer in Asia.4) The hydrocarbon sesquiterpenoids in spikenard, such as tricyclic aristolane type compounds and bicyclic valerena-4,7(11)-diene (VLD), were identified as the active sedative ingredients.5) Among these compounds, VLD showed a particularly profound sedative effect (Fig. 1). Currently, the presence of this compound has only been reported in plants of the Valerianaceae Valeriana species6) and Nardostachys families.7)

Stressful events give rise to several neurochemical changes that promote emotional and behavioral responses, including sympathetic activities that might facilitate an organism’s ability to deal with the stressors and ameliorate adverse consequences.8) Sabban et al. reported that restraint stress induces overactivation of the sympathetic nervous system in rats, and mean arterial pressure and heart rate reached a peak value within 15 min after loading stress.9) Along with other biological responses, stressors activate the hypothalamic–pituitary–adrenal (HPA) axis and increase the release of monoamines into the hippocampus, hypothalamus, and striatum, which are brain regions that have been associated with anxiety and depression.10) Notwithstanding adaptive neurochemical changes, sufficient intensity and persistence of stressors may place excessive strain on these biological processes, leading to allostatic overload and the development of psychological and physical disorders.11)
In the present study, we evaluated VLD as an anti-stress agent. We used restraint stress for 15 min as a model of acute stress and investigated the effects of inhaled VLD on acute stress-related changes in mice behaviors and stress-related factors (serum corticosterone and cerebral monoamines). Subsequently, we identified pathways that mediate the effects of inhaled VLD. It is known that inhaled volatile constituents act on the central nervous system through olfactory nerves and the bloodstream.12) Thus, we investigated the effects of VLD inhalation on anosmic mice, and compared tissue transitions following inhalation and intravenous (i.v.) administration of VLD.
VLD was dissolved in the odorless solvent triethylcitrate (purity >99%, GC; Wako Pure Chemical Industries, Ltd., Osaka, Japan). Caffeine (purity >98.5%, TLC) was purchased from Wako Pure Chemical Industries, Ltd., diazepam was obtained from Taiyo Yakuhin Co., Ltd. (Tokyo, Japan), chlorpromazine hydrochloride (purity >98%, TLC) was obtained from Sigma-Aldrich, Inc. (St. Louis, MO, U.S.A.), and pentobarbital sodium salt (purity >98%) was purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Caffeine was dissolved in distilled water, diazepam in 1% CMC-Na (aq.), and pentobarbital and chlorpromazine in physiological saline. Column chromatography was performed using a 60 N silica gel (40–50 µm, Kanto Chemical Co., Ltd., Tokyo, Japan). Preparative HPLC was performed using a Cosmosil® 5C18-AR-2 column (150 mm×20 mm i.d.; Nacalai Tesque, Inc., Kyoto, Japan) and the compound was detected using ultraviolet (SPD-10A, Shimadzu Corporation, Kyoto, Japan) and refractive index measurements (RI-72, SHOWA DENKO K.K., Tokyo, Japan). All chemicals and reagents were of the highest grade available.
Plant MaterialSpikenard (lot number: 0080) was purchased from Mitsuboshi Pharmaceutical Co., Ltd. (Nara, Japan) and identified by the authors. A voucher specimen of the plant material (No. 3735) was deposited in the Herbarium of Medicinal Plant Garden, Tokyo Metropolitan Institute of Public Health (Tokyo, Japan).
VLD IsolationDried roots of N. chinensis (150 g) were subjected to acetone extraction three times at room temperature. The acetone extract was concentrated under reduced pressure, and silica gel column chromatography (25×5 cm) was performed on the concentrate (12 g). The hexane eluting fraction (246.9 mg) was applied to preparative HPLC and was eluted in CH3CN–H2O=95 : 5 (flow rate, 8 mL/min) to give VLD (43.0 mg, purity >99%, GC-FID). Spectral data agreed with those from previous studies.6)
Spectral DataValerena-4,7(11)-diene; [α]D25.0 −7.3° (c=0.10, CHCl3); 13C-NMR (CDCl3) δ: 12.0, 13.3, 17.7, 24.5, 26.0, 26.5, 28.6, 33.5, 33.6, 37.5, 47.4, 126.3, 128.4, 129.8, 136.1. Electron ionization (EI)-MS (GC-MS) 70 eV, m/z (rel. int.): 204 [M]+ (100), 189 [M−Me]+ (63), 161 (67), 147 (60).
AnimalsFour-week-old male ddY mice were purchased from Japan SLC, Inc. (Shizuoka, Japan). Prior to experimentation, mice were acclimatized for 1 week to a temperature of 25±2°C, humidity of 50±10%, and a 12-h light/12-h dark cycle. All behavioral observations were conducted between 10:00 and 15:00 h. Experiments were performed in accordance with the Kitasato University guidelines for animal care, handling, and termination, which are in line with the international and Japanese guidelines for animal care and welfare (approval number: FR07-2, date: May 1, 2013).
Administration by InhalationInhalation treatments were performed according to the method of Kobayashi et al.13) VLD was dissolved in triethylcitrate (30 µL total) prior to use. Thick paper disks (2.5×3.0 cm, 1-mm thickness) were permeated with sample solution and were placed on a hotplate diffuser (ca. 70°C) in the upper portion of the cage. The cylindrical cage was made of transparent polycarbonate (22 cm in height, 25 cm in diameter) and was covered with a vinyl film to ensure perfusion of the sample vapors in the cage. Thirty minutes after charging the solution, a mouse was placed into the center of the cage. VLD inhalation doses were set to 30 or 300 µg/cage based on previous results.5)
Stress LoadingAfter fasting for 2 h, mice were subjected to restraint stress for 15 min using a stress cage (Natsume Seisakusho Co., Ltd., Tokyo, Japan).
Forced Swim TestThe forced swim test was performed according to the method of Kobayashi et al.14) with minor modifications. The same cylindrical cages were used as water tanks for swimming (water depth 15 cm, water temperature 25°C). Twenty-four hours before experiments, mice were individually placed in the water tank for 15 min, and were grouped according to measurements of immobility times. The influence of stress on swimming was compared with that of orally administered caffeine (20 mg/kg). To evaluate the stress-relieving effects of VLD, the water tank was covered with a vinyl film and the air in the tank was filled with vapors from test compounds. Stressed mice were individually placed into the water tank and immobility times were measured over 6 min. Fifteen minutes prior to stress loading, diazepam (5 mg/kg) was administered orally for comparison with VLD inhalation. Immobility times of each mouse were measured individually using a SUPERMEX (Muromachi Kikai Co., Ltd., Tokyo, Japan), and data were analyzed using a personal computer with the CompACT FSS software (Muromachi Kikai Co., Ltd.).
Pentobarbital Sleep TestThe influence of stress on sleeping was compared with that of orally administered caffeine (20 mg/kg). Mice were given intraperitoneal (i.p.) injections of 30-mg/kg pentobarbital upon termination of stress treatments, and were then placed in a cage filled with sample solution vapor. The sleep duration was defined as the difference in time between the loss and the recovery of the righting reflex. Fifteen minutes prior to stress loading, chlorpromazine was administered orally (5 mg/kg) for comparison with inhaled VLD.
Measurements of Serum Corticosterone and Cerebral Monoamine LevelsMice were divided into control, stress, VLD, and vehicle groups. Mice were subjected to restraint stress alone, and VLD and vehicle groups inhaled VLD or vehicle for 30 min after restraint stress loading. Subsequently, whole blood was collected from the femoral artery of each mouse under ether anesthesia, and was centrifuged at 1500×g for 10 min at 4°C to separate sera. Serum corticosterone levels were measured using a commercial Corticosterone ELISA kit (Assaypro LLC, St. Charles, MO, U.S.A.), according to the manufacturer’s instructions.
Hypothalamus and striatum tissues were separated and weighed. Tissues were then fractured in a 0.2-M perchloric acid solution containing 0.1-mM ethylenediamine tetraacetic acid (EDTA) and isoproterenol as internal standards. Homogenates were then centrifuged at 20000×g for 15 min. Supernatants were adjusted to pH 2.9 using 1-M sodium acetate and were then passed through a 0.45-µm filter. Aliquots of the resulting filtrates were injected onto a C18 reversed-phased column (Inertsil® ODS-3, GL Sciences Inc., Tokyo, Japan) in a HPLC system equipped with an electrochemical detector (NANOSPACE®SI-2, Shiseido Co., Ltd., Tokyo, Japan). The conditioning cell was set at +700 mV. The mobile phase used with these aliquots (0.1-M phosphate buffer containing 5% acetonitrile) allowed separation of the three major monoamines norepinephrine (NE), dopamine (DA), and serotonin (5-HT), and their metabolites 3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), and 5-hydroxyindoleacetic acid (5-HIAA). 1-Octanesulfonic acid sodium salt (0.7 mM) was added as an ion-pairing agent, and EDTA (0.02 mM) was added as an antioxidant.
Induction of AnosmiaAnosmia was induced in mice according to the method of Chuah et al.15) with minor modifications. In brief, intranasal irrigation of the bilateral nose with 5 µL of aqueous solution containing 48.9-mg/mL ZnSO4 was performed slowly under mild anesthesia with pentobarbital. This treatment was performed once daily for 5 successive days. According to the method described by Kobayashi and Bhatt,16) the open field test was performed 2 d after termination of the induction period in the same cylindrical cages equipped with a passive infrared sensor (PYS-001, Muromachi Kikai Co., Ltd.). Spontaneous motor activity was recorded using a passive infrared sensor detection system (SUPERMEX, Muromachi Kikai Co., Ltd.) and data were analyzed using CompACT AMS software (Muromachi Kikai Co., Ltd.).
Measurement of Blood and Whole Brain Concentrations of VLDSamples of blood were collected from the jugular vein under ether anesthesia after 15-, 30-, or 60-min inhalation periods (300 µg/cage), and whole brain samples were collected after perfusion with physiological saline from the left ventricle. For each sample, VLD was extracted using diethyl ether with an ultrasonic homogenizer. Diethyl ether suspensions were centrifuged at 20000×g for 10 min at 4°C, and supernatants were concentrated in vacuo. Residues were dissolved in acetone containing 6.2×10−4-mM d-limonene as an internal standard, applied to an octadecyl silica (ODS) flash column, and eluted with 4 mL of CH3CN. The resulting fractions were evaporated to dryness, and 1 µL of acetone solutions of residue were injected into the GC-MS system. The peak areas at m/z 136 for d-limonene and at m/z 204 for VLD were calculated from mass chromatograms.
GC-MS AnalysisGC-MS analyses were performed using a GC-2010/GCMS-QP2010 Plus instrument (Shimadzu Corporation) fitted with a 30-m×0.25-mm Rtx®-5MS column of 0.25-µm film thickness (Restek Corporation, Bellefonte, PA, U.S.A.). Typical analysis was performed under the following conditions: column temperature program: 60°C (1 min hold)→180°C (Δ2°C/min)→280°C (Δ10°C/min, 15 min hold); carrier gas: helium (flow rate 38.3 cm/s); injector: 230°C; interface: 250°C; ion source: 200°C; and injection volume: 1 µL (split less). The MS instrument was operated in the EI mode at an ionization voltage of 70 eV over an m/z range of 30–400 amu.
Intravenous Administration TestVLD was suspended in physiological saline containing 0.1% CMC-Na and was administered to mice i.v. at doses of 10, 100, and 1000 µg/kg. Fifteen minutes after administration, each mouse was placed in the center of the cage and total motor activity was measured for another 60 min.
Statistical AnalysisAll results are expressed as the mean±standard deviation (S.D.). The data were analyzed using the one-way ANOVA followed by the Tukey’s multiple comparison test. In the experiment of Fig. 5, Student’s t-test was used to compare the values from two groups. All statistical analyses were performed using Prism 5 (GraphPad Software Inc., San Diego, CA, U.S.A.) and differences were considered significant when p<0.05.
Mice were subjected to restraint stresses for 15 min, and changes in their behavior were investigated in open field, forced swim, and pentobarbital sleep tests. Stress had no effect on locomotor activities in the open field test (data not shown). However, significant effects were observed in the forced swim and pentobarbital sleep tests. Stress treatments shortened immobility times by 43% (Fig. 2) in the forced swim test and sleeping times by 47% (Fig. 3) in the pentobarbital sleep test compared with those of the control mice. Reductions in immobility and sleeping times were similar to the effects of orally administered caffeine, which decreased immobility times by 56% (Fig. 2) and sleeping times by 61% (Fig. 3). Inhalation of VLD significantly suppressed stress-induced behavioral changes. In the forced swim test, immobility times were prolonged in a dose-dependent manner (Fig. 2) to 173 and 254 s by doses of 30 and 300 µg/cage, respectively. Moreover, in the pentobarbital sleep test, sleeping times were also prolonged in a dose-dependent manner (Fig. 3) to 24 min and 31 min by doses of 30 and 300 µg/cage, respectively. These observations indicate that VLD (300 µg/cage) completely prevented stress-induced behavioral changes in both tests. The effects of VLD were comparable with those of the benzodiazepine receptor agonist diazepam and the D2-receptor antagonist chlorpromazine (Figs. 2, 3).

Data are presented as the mean±S.D. of each group (n=8). Comparisons were made using one-way ANOVA followed by Tukey’s test; ** p<0.01 vs. control group, # p<0.05, ## p<0.01 vs. vehicle group, †† p<0.01 vs. stress group.
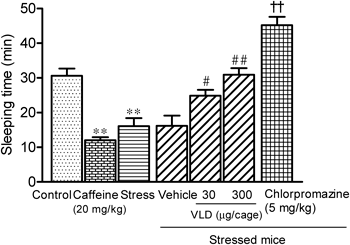
Data are presented as the mean±S.D. of each group (n=8). Comparisons were made using one-way ANOVA followed by Tukey’s test; ** p<0.01 vs. control group, # p<0.05, ## p<0.01 vs. vehicle group, †† p<0.01 vs. stress group.
The relaxing effects of VLD on the HPA axis were evaluated by measuring serum corticosterone (Fig. 4), a well-known rodent stress marker.17) The mean corticosterone level in the stress group was 2.74 ng/mL, almost double of that in the control group (1.57 ng/mL). Blood corticosterone levels in the vehicle group were decreased 30 min after termination of restraint stress but remained elevated (2.29 ng/mL). In contrast, administration of 300 µg/cage VLD vapor significantly reduced the blood corticosterone level to 1.41 ng/mL, which was almost equivalent to that of the control group.

Data are presented as the mean±S.D. of each group (n=6). Comparisons were made using one-way ANOVA followed by Tukey’s test; ** p<0.01 vs. control group, ## p<0.01 vs. vehicle group.
Concentrations of cerebral monoamines and metabolites are shown in Table 1. No significant changes in NE, DOPAC, or 5-HIAA concentrations were observed in either the hypothalamus or the striatum region after stress treatment. However, DA and 5-HT levels were significantly elevated by restraint stress. DA concentrations in the striatum were significantly increased by stress, and high levels were also observed in the vehicle-treated mice. In addition, increases in DA concentrations were observed in the hypothalamus significantly at 30 min after termination of restraint stress (vehicle group). Similar to fluctuations of DA, restraint stress induced significant increases in the concentration of 5-HT in both regions, and high levels were also observed in the vehicle group in the striatum. Stress also caused significant changes in the corresponding amine/metabolite ratios in each region. In the hypothalamus, DOPAC/DA ratios were decreased in the vehicle group, and 5-HIAA/5-HT ratios were decreased in the stress group. In the striatum, DOPAC/DA ratios were decreased in the stress group, which potentially reflected declines in DA and 5-HT metabolic activity due to restraint stress.18) VLD inhalation significantly attenuated increases in cerebral 5-HT and DA levels and also decreased DA turnover in the hypothalamus and striatum after restraint stress.
| Brain region | Groups | Monoamine and metabolite (ng/mg weight tissue) | Ratio | ||||||
|---|---|---|---|---|---|---|---|---|---|
| NE | DA | DOPAC | HVA | 5-HT | 5-HIAA | DOPAC/DA | 5-HIAA/5-HT | ||
| Hypothalamus | Control | 2.141±0.110 | 0.403±0.076 | 0.089±0.020 | 1.736±0.292 | 0.976±0.120 | 0.400±0.037 | 0.232±0.086 | 0.415±0.066 |
| Stress | 2.032±0.141 | 0.463±0.091 | 0.094±0.004 | 2.288±0.326* | 1.134±0.122* | 0.379±0.022 | 0.209±0.038 | 0.336±0.021* | |
| Vehicle | 2.058±0.256 | 0.620±0.199** | 0.102±0.032 | 1.926±0.706* | 0.979±0.131 | 0.408±0.105 | 0.188±0.026* | 0.415±0.077 | |
| VLD | 2.050±0.230 | 0.432±0.083## | 0.098±0.015 | 2.022±0.315 | 1.060±0.080 | 0.440±0.053 | 0.241±0.099# | 0.415±0.034 | |
| Striatum | Control | 0.228±0.031 | 5.389±0.471 | 0.379±0.092 | 7.870±0.284 | 0.448±0.044 | 0.170±0.028 | 0.064±0.006 | 0.379±0.050 |
| Stress | 0.228±0.033 | 6.336±0.677** | 0.288±0.094 | 7.788±1.560 | 0.534±0.043** | 0.180±0.029 | 0.045±0.010** | 0.355±0.038 | |
| Vehicle | 0.239±0.015 | 6.353±0.437** | 0.352±0.053 | 7.689±0.403 | 0.532±0.046** | 0.203±0.014* | 0.055±0.006 | 0.383±0.026 | |
| VLD | 0.226±0.057 | 5.841±0.227# | 0.362±0.078 | 7.732±0.804 | 0.486±0.021# | 0.201±0.038 | 0.057±0.007 | 0.414±0.068 | |
Results represent the mean±S.D. of each group (n=6). Comparisons were made using one-way ANOVA followed by Tukey’s test; * p<0.05, ** p<0.01 vs. control group, # p<0.05, ## p<0.05 vs. vehicle group.
The open field test was performed in the control and anosmic mice in a cage filled with 300 µg/cage VLD vapor (Fig. 5). In the control group, locomotor activity was inhibited by 55%, but it was only inhibited by 27% in the anosmic mice.

Data are presented as the mean±S.D. of each group (n=6). Statistical differences vs. vehicle group indicated by * p<0.05, ** p<0.01 (Student’s t-test).
Mean VLD concentrations in blood and brain tissues after 15-, 30-, or 60-min inhalation periods are shown in Table 2. VLD was detected in blood and brain samples after 15-min inhalation, and VLD concentrations in the brain reached a plateau after 30-min inhalation, whereas those in the blood continued to increase in an inhalation time-dependent manner.
| Time after administration (min) | Inhalation administration | i.v. administration | |||
|---|---|---|---|---|---|
| Whole brain (ng/g weight tissue) | Blood (ng/mL) | Blood (ng/mL) | |||
| 10 µg/kg | 100 µg/kg | 1000 µg/kg | |||
| 15 | 5.62±0.17 | 4.04±0.14 | nd | 2.95±0.45 | 33.9±0.29 |
| 30 | 11.1±0.27 | 5.01±0.32 | |||
| 60 | 10.9±0.25 | 8.34±0.35 | |||
Data are presented as the mean±S.D. of each group (n=6). nd, not detected.
VLD was administered intravenously at doses of 10, 100, and 1000 µg/kg, and open field tests were performed (Fig. 6). The total motor activity counts in mice administered 10 and 100 µg/kg of VLD were 3136 and 1861, respectively, compared with 4056 in the vehicle group. Sedative effects were greater in the 100 µg/kg group than in the 10 µg/kg group; however, treatment with 1000 µg/kg caused a decreasing effect (3165 counts), suggesting an overdose.

Data are presented as the mean±S.D. of each group (n=8). Comparisons were made using one-way ANOVA followed by Tukey’s test; ** p<0.01 vs. vehicle group.
Spikenard has been used as a traditional herbal tranquilizer and is abundant in sesquiterpenoids. Inhalation administration is one of the non-invasive routes; therefore, we investigated the sedative effect of volatile compounds to assess the effectiveness of spikenard as aromatherapy. We previously reported that aristolane-type calarene, which is the main component (13.5%) in spikenard essential oil, showed a sedative effect by inhalation administration. However, minor component VLD (1.9%) showed a particularly profound sedative effect. VLD is a characteristic compound in plants of the Valerianaceae family. Therefore, in this study, behavioral pharmacological and biochemical analysis using stress-loaded mice were performed to investigate the stress-reducing effect of VLD.
Application of 15-min restraint stress induced significant changes in mice, including shortening of immobility and sleeping time, increases in serum corticosterone levels, increases in brain DA and 5-HT levels, and declines in DA and 5-HT metabolic activities. Reductions in immobility and sleeping times were similar to the effects of orally administered caffeine, suggesting that stress induced an excitatory state in mice. Sabban et al. also reported that elevation of mean arterial pressure and heart rate of rats reached a peak within 15 min after loading restraint stress.9) From our finding of monoamine fluctuation, the excitatory states of mice after restraint stress may reflect increases in DA activity and activation of the sympathetic nervous system. Furthermore, activation of the HPA axis was also observed, which suggested that acute stress reaction was induced by 15-min restraint stress. However, only small differences in the increased DA and 5-HT levels were observed. In the hypothalamus, increases in the 5-HT level occurred before increases in the DA level. It has been reported that the serotonergic system plays a pivotal role in regulating the activity of the HPA axis.19) Moreover, rapid increases in serotonergic activity after acute stress reportedly occur before levels of other catecholamines become elevated, suggesting that the serotonin system may play a key role in triggering the initial steps of HPA-axis activation20) and DA release.
Inhalation administration of VLD significantly ameliorated restraint stress-induced changes in excited behaviors and stress-related factors. It is suggested that prolongation of shortened immobility and sleeping time was caused by sedation of mice in the stress-induced excitatory state. The sedative mechanism of VLD was estimated from the results of the diazepam and chlorpromazine groups, which showed reactions similar to those of the VLD group. It is known that benzodiazepine affects 5-HT regulation and diazepam administration reduces 5-HT synthesis21) and inhibits 5-HT-induced corticotropin-releasing hormone secretion.22) Reduction of 5-HT levels by VLD administration appears to precede suppression of the HPA axis. Valerenic acid, an analog of VLD, is a major constituent of Valeriana officinalis, and Kohm et al. have identified valerenic acid as a subunit-specific modulator of γ-aminobytyric acid type A(GABAA) receptors.23) Hence, there is a possibility that the tranquilizing effects of VLD appear to mainly involve GABAergic activity. In another report, social defeat stress initiated depression and decreased DA turnover in rats, and the D2 antagonist, amisulpride, prevented these defeat-induced reductions in DA turnover.24) Attenuation of dopaminergic neurons directly may lead to sedation of excitement behaviors and sympathetic activities. Therefore, it also seemed that D2-antagonist activity was involved in the effect of VLD.
Moreover, in this study, we identified pathways that mediated the effects of inhaled VLD. Either olfactory stimulation or pulmonary absorption is responsible for the effects of pulmonary administered volatile compounds. The sedative effect of VLD significantly decreased in anosmic mice, and VLD was detected in blood and brain samples after inhalation. These results suggest that the sedative effects of VLD follow olfactory stimulation and pulmonary absorption. To confirm the involvement of pulmonary absorption, VLD was administered intravenously, and significant effect was observed in the 100 µg/kg group. Notably, blood VLD concentrations in the 300 µg/cage group were similar to those in the mice treated i.v. with 100 µg/kg VLD (Table 2), indicating that circulating concentrations produced sedative effects.
VLD is a natural oriental herbal medicine with a pleasant smell. The results of the present study suggest that the compound could be useful as an anti-stress agent for treatment of various mental disorders. Further studies are required to elucidate the underlying mechanisms and other characteristics of this compound; however, this volatile compound may have significant potential for future development.