2015 年 38 巻 11 号 p. 1681-1688
2015 年 38 巻 11 号 p. 1681-1688
Irsogladine maleate (2,4-diamino-6-[2,5-dichlorophenyl]-s-triazine maleate; IM), an anti-peptic ulcer drug, may have a protective effect on the gastrointestinal mucosa. This study investigated the effects of IM on spontaneous colitis in interleukin-10 gene-deficient (IL-10−/−) mice. Five-week-old IL-10−/− mice were fed a control diet or one containing 100 ppm of IM for 10 weeks. Colonic tissues were evaluated morphologically and histologically. J774A.1 murine monocyte/macrophage cells were incubated with IM after lipopolysaccharide stimulation. mRNA expression was assessed by quantitative polymerase chain reaction (PCR) and protein concentration by enzyme-linked immunosorbent assay (ELISA). Colonic length, weight, and histological scores clearly demonstrated that spontaneous colitis was prevented in IL-10−/− mice fed a diet containing IM compared with those fed control diet. Levels of tumor necrosis factor-alpha (TNF-α) (−2.5-fold), IL-1β (−5.4), interferon-gamma (IFN-γ) (−4.5), IL-17 (−113.0), IL-12p35 (−21.0), IL-12p40 (−3.4), and IL-23p19 (−4.2) mRNA expression were significantly decreased in the colonic tissues of IM-treated animals, suggesting that oral treatment with IM suppressed the T-helper (Th)1/Th17 immune response in the colonic mucosa. An in vitro study using monocyte/macrophage cells to clarify the pharmacological action of IM indicated that IL-12p40 and IL-23p19 mRNA expression levels were dose-dependently decreased by IM treatment. ELISA showed that IL-12p40 and IL-23 protein secretion were significantly decreased by IM in a dose-dependent manner. Oral treatment with IM prevented spontaneous colitis in IL-10−/− mice by suppressing the colonic mucosal Th1/Th17 immune response through inhibition of IL-12 and -23 production in monocyte/macrophage cells.
Inflammatory bowel diseases (IBD) are chronic inflammatory disorders of the gastrointestinal tract. The current paradigm of IBD pathogenesis suggests aberrant activation of innate and adaptive immune responses in which aggressive mucosal CD4+ T-helper (Th) cells play a major role, leading to tissue damage and loss of immune homeostasis.1–4) A number of recent studies have demonstrated the importance of the Th1/Th17 axis in the pathogenesis of IBD.5)
The interleukin 10 gene-deficient (IL-10−/−) mouse is a well-established spontaneous murine model of IBD.6,7) The colitis of IL10−/− mice is characterized by mononuclear infiltration of the colonic lamina propria and enhanced secretion of IL-12/23p40.8,9) Intestinal macrophages in IL-10−/− mice have been shown to differentiate into an abnormal phenotype, which leads to Th1/Th17-dominant colitis via hyperproduction of IL-12 and IL-23 in the presence of bacteria.10) Moreover, Yen et al. demonstrated that IL-23 was essential for the manifestation of chronic intestinal inflammation in IL-10−/− mice. They also demonstrated that tissue-homing memory T cells were specifically activated by IL-23 to produce the proinflammatory mediators IL-17 and IL-6.11) Therefore, utilizing IL-10−/− mice as a model of human chronic intestinal inflammation was appropriate for evaluating disorder of the Th1/Th17 axis.
Irsogladine maleate [2,4-diamino-6-(2,5-dichlorophenyl)-s-triazine maleate] (IM) is a well-tolerated drug that has been used for treatment of peptic ulcers for several decades.12–14) Several recent reports have indicated that IM has antiinflammatory and protective effects in areas of the gastrointestinal mucosae other than the stomach.15,16) The administration of IM by enema was shown to improve chronic experimental colitis induced by dextran sulfate sodium, suggesting that topical IM therapy could downregulate cytokine production.15) The present study was designed to investigate the effects of oral administration of IM on spontaneous colitis in the IL-10−/− mouse.
Homozygous interleukin-10 gene-deficient (IL-10−/−) mice and C57BL/6 (B6) mice were obtained from Charles River Japan, and were housed under specific pathogen-free conditions at the Laboratory Animal Center of Chiba University after quarantine and acclimation. Wild-type littermates generated from IL-10−/− and B6 mice were also maintained at the above facility, and were fed normal animal chow with free access to tap water. Wild-type littermates were genotyped according to the protocol of the Jackson Laboratory (http://jaxmice.jax.org/strain/002251.html).
Growth retardation of IL-10−/− mice becomes evident between 3 and 4 weeks of age, and approximately 90% of the mice show anemia at 7–11 weeks old because of spontaneous colitis.6) Therefore, we used 40 IL-10−/− mice at the age of 5 weeks old to validate the prophylactic antiinflammatory effect of IM, and equally assigned them to either a control group (given a normal diet) or an IM group (given a diet containing 100 ppm of IM), taking gender ratio into consideration, for 10 consecutive weeks. The IM diet consisted of chow mixed with 100 ppm of IM synthesized by Nippon Shinyaku Co. (Kyoto, Japan). The IM concentration in the diet was decided based on the difference in human and rodent plasma level of MN-1695 (Irsogladine maleate [2,4-diamino-6-(2,5-dichlorophenyl)-s-triazine maleate]). In a phase I study of MN-1695, the half-life mean of the plasma level in human subjects was 145 h,17) while that in rats was 2–3 h in a preclinical study.18) In addition, the approved dose of IM for patients with peptic ulcer or gastritis in Japan is 4 mg/d. Therefore, the administration of 0.2–0.4 mg of IM daily to the mice was considered to be a clinically relevant dose. All animals were checked for overall condition, weighed weekly, and euthanized on day 70 (after 10 weeks on the experimental diets; 15 weeks of age). The intestines of the IL-10−/− mice and their wild-type littermates were extracted and the length and the weight of the colon were measured after removing stools with phosphate buffered saline (PBS). All experimental procedures were approved by the Institutional Animal Care and Use Committee of Chiba University.
Morphology and HistologyThe colons were removed from euthanized animals and washed with PBS. The length and weight of the colon were measured, and gut tissues were fixed in 4% phosphate buffered formalin, and embedded in paraffin for sectioning, followed by hematoxylin and eosin (H&E) staining for histological analyses. The slides were reviewed in a blinded manner and were assigned a histological score for intestinal inflammation as described previously19,20) (Table 1). The mean and standard error of the mean (S.E.M.) of the score for each mouse were determined by adding the score for each section of the colon examined (minimum of three sections per mouse) and dividing the total by the number of sections examined.
Cells and Preparation of Cell SuspensionsThe murine monocyte/macrophage cell line J774A.1 from the American Type Culture Collection (Manassas, VA, U.S.A.) was cultured in RPMI-1640 medium (GIBCO/Invitrogen, Carlsbad, CA, U.S.A.) supplemented with 50 U/mL of penicillin/streptomycin and 10% heat-inactivated fetal bovine serum (GIBCO/Invitrogen) in an incubator at 37°C with 5% CO2. After overnight cell culture in 6-well plates at 0.5×106 cells/well, 100 ng/mL of lipopolysaccharide (LPS; InvivoGen, San Diego, CA, U.S.A.) was added to each well to stimulate IL-12p40 as described previously.21) IM can be dissolved easily in 100% ethanol, but not in water. Therefore, to prepare IM solutions at a final concentration ranging from 10−4 to 10−7 M in cell medium, 100-fold concentrated IM solutions were added to each well. The same volume of 100% ethanol as IM solution was added to control wells. The cells were scraped after 24-h incubation, and washed with PBS prior to extraction of total RNA.
RNA Isolation and Quantitative Reverse Transcription Polymerase Chain Reaction (RT-PCR) (qPCR)Proximal colon and ileum tissue samples were washed with PBS and stored in RNAlater (Qiagen, Hilden, Germany). The J774A.1 cell suspensions were prepared as described above. Total RNA was extracted using a QIAshredder and RNeasy Mini Kit according to the manufacturer’s instructions (Qiagen). Reverse transcription (RT) of the extracted RNA was performed using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, U.S.A.). RT was carried out on 100 ng of extracted total RNA with incubation at 25°C for 10 min, 37°C for 120 min, and 85°C for 5 min on a GeneAmp PCR System 9700 Thermal Cycler (Applied Biosystems). The qPCR assay was an RNA-specific predesigned TaqMan Gene Expression Assay from Applied Biosystems [tumor necrosis factor-α (TNF-α): Mm00443258_m1; interleukin-1β (IL-1β): Mm001336189_m1; interferon-γ (IFN-γ): Mm99999071_m1; interleukin 17A (IL-17): Mm00439618_m1; interleukin-12a (IL-12a: IL-12p35): Mm00434165_m1; interleukin-12b (IL-12b: IL-12p40): Mm00434174_m1; interleukin-23a (IL-23a: IL-23p19): Mm00518984_m1], and the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene was used as an internal standard (Mm99999915_g1). All qPCR cycle threshold (Ct) data were obtained in duplicate with all samples in triplicate. Samples were analyzed using StepOne software, and cytokine expression levels were determined using the comparative Ct method (ΔΔCt method). The values were calculated as Relative Quantification (RQ) standardized relative to wild-type littermates.
Measurement of Cytokine Production by Enzyme-Linked Immunosorbent Assay (ELISA)Using the same method as described for preparation of cell suspensions, the cell supernatants were collected to determine TNF-α, IFN-γ, IL-12p40, and IL-23 production after stimulation with LPS and treatment with irsogladine in a dose-dependent manner. ELISA was performed using TNF-α, IFN-γ, and IL-12p40 Mouse ELISA Kits (Thermo Fisher Scientific, Waltham, MA, U.S.A.) and an IL-23 Mouse ELISA Kit Quantikine (R&D Systems, Minneapolis, MN, U.S.A.) according to the respective manufacturer’s instructions. All ELISA experiments were performed in duplicate with all samples in triplicate.
Statistical AnalysisQuantitative values are expressed as means and S.E.M. Data were tested for normality of distribution, and analyses were performed using SPSS version 22 (IBM, Armonk, NY, U.S.A.). Specific differences were tested using Student’s t-test and Dunnett’s test if more than two variables were considered. In all analyses, p<0.05 was taken to indicate statistical significance.
Forty IL-10−/− mice at the age of 5 weeks old were assigned to either a control group or an IM group, as described in Materials and Methods. During the observation period, no deaths or pregnancies were seen in any mice. Macroscopic colonic findings at 15 weeks of age showed wall thickening and redness due to severe colitis in colon tissue samples from IL-10−/− mice fed the control diet, which were similar to previous reports regarding the morphological features of IL-10−/− mice.6) Notably, in the colon of IL-10−/− mice fed with IM, only a few red spots were found and the colonic wall thickness was comparable with that of wild-type littermates (Fig. 1A). The mean length of the colon in IL-10−/− mice fed with IM was significantly longer than that of IL-10−/− mice fed the control diet. Moreover, the mean weight of the colon from the IL-10−/− mice fed IM was significantly less than that of IL-10−/− mice in the control group (Fig. 1B). These findings clearly indicated prevention of spontaneous colitis in IL-10−/− mice by oral treatment with IM.

The upper panel shows the entire colon from each of three IL-10−/− mice fed a control diet, and the lower panel shows the entire colon from each of four IL-10−/− mice fed the same diet containing IM (IM group). Each colon was resected from the anal side to the cecum. The scale at the bottom of each panel is in centimeters.
(B) Comparison of Colon Length and Weight between Control and IM Groups
Data from control and IM groups are indicated by gray and black bars, respectively, and are expressed as mean±S.E.M. Student’s t-test was used for statistical analysis.
Significant wall thickening, formation of enlarged and branched crypts, and goblet cell depletion with apparent inflammatory cell infiltration were observed in IL-10−/− mice fed the control diet (Fig. 2A). In contrast, colon specimens from IL-10−/− mice treated with IM showed no abnormal crypt formation or goblet cell depletion, and there were marked reductions of both colonic wall thickening and inflammatory cell infiltration compared with IL-10−/− mice fed the control diet (Fig. 2B). The mean histological score (Table 1) in the control group was 3.8±0.1 due to mucosal hyperplasia, mononuclear infiltrates in the lamina propria, loss of goblet cells, and occasional crypt abscesses, crypt ulcers, and transmural inflammation. However, the score of the IM group (1.6±0.1) was significantly lower than that of the control group due to the decreased mucosal thickening and goblet cell depletion (Fig. 2C), further evidence that oral IM treatment significantly reduced colonic inflammation in IL-10−/− mice.

Colonic specimens were stained with hematoxylin and eosin to evaluate the presence of inflammatory cells and the mucosal architecture. The top is the luminal surface with original magnification ×40 and ×250.
(C) Quantitative Evaluation of Histological Findings Using the Histological Scores in Table 1
Scores of IL-10−/− control mice and IL-10−/− IM mice are indicated by gray and black bars, respectively. Data are expressed as mean±S.E.M. Student’s t-test was used for statistical analysis.
| Criterion | Score | Add | ||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | +1 for each | |
| Inflammatory cells | − | + | ++ | +++ | +++ | Ulcer |
| Goblet cell depletion | − | + | ++ | +++ | +++ | Crypt abscess |
| Mucosal thickening | − | + | ++ | +++ | +++ | |
| Submucosal cell infiltration | − | − | + | ++ | +++ | |
| Destruction of architecture | − | − | − | + | ++ | |
The above scores for each section were determined according to the criteria on the left. The scores for sections with positive findings for ulcer or crypt abscess were given 1 point for each finding. The histological score of each mouse was determined as the average of the scores for each intestinal section.
Forty IL-10−/− mice at the age of 5 weeks old were divided equally into a control group that was given the normal diet and an IM group given the same diet containing 100 ppm irsogladine for 10 consecutive weeks as described in Materials and Methods. During the observation period, no deaths or pregnancies were found in any groups of mice and there were no differences in growth curves between the IM and control groups on weekly records (Figs. 3A, B).

Forty IL-10−/− mice at the age of 5 weeks old were divided equally into a control group that was given normal diet and an IM group given the same diet containing 100 ppm irsogladine for 10 consecutive weeks as described in Materials and Methods. During the observation period, no deaths or pregnancies were found in any group. Data from control and IM groups are indicated by open and closed circles, respectively.
(B) Comparison of Body Weight between Control and IM Groups at 15 Weeks of Age
Data from control and IM groups are indicated by gray and black bars, respectively, and are expressed as mean±S.E.M. Student’s t-test was used for statistical analysis.
Previous studies have demonstrated that large amounts of proinflammatory cytokines are produced in IL-10−/− mice.22,23) To evaluate mRNA expression levels of cytokines, such as TNF-α, IL-1β, IFN-γ, and IL-17, qPCR analyses were performed using mRNA extracted from colonic tissues. TNF-α, IL-1β, and IFN-γ mRNA expression levels in the colonic tissues of IL-10−/− mice were significantly higher (16-, 64-, and 29-fold, respectively) than those of wild-type littermates (Figs. 4A–C), suggesting that the immune response of IL-10−/− mice is at least partly mediated by the Th1 pathway. The expression levels of TNF-α, IL-1β, and IFN-γ mRNA in the colon tissue from IL-10−/− mice treated with IM were significantly decreased in comparison to IL-10−/− mice fed the control diet (Figs. 4A–C). Furthermore, IL-17 mRNA expression was significantly increased in IL-10−/− mice (113.0-fold) compared with wild-type littermates, suggesting that the immune response of IL-10−/− mice is partly mediated by the Th17 pathway. IL-17 mRNA expression was significantly reduced in IL-10−/− mice treated with IM (Fig. 4D).

All panels show wild-type littermates as white, control mice as gray, and mice treated with IM as black bars. The scales on the left side of the graphs indicate relative quantification compared with mRNA expression in wild-type littermates. Comparisons of colonic TNF-α (A), IL-1β (B), IFN-γ (C), IL-17 (D), IL-12p35 (E), IL-12p40 (F), and IL-23p19 (G) expression between control and IM groups are shown. Data are expressed as mean±Student’s t-test was used for statistical analysis.
To further investigate the effects of IM in suppressing spontaneous colitis in IL-10−/− mice, we focused on IL-12 and IL-23, the upstream cytokines of the Th1 and Th17 pathways. IL-12 (which regulates IFN-γ secretion in Th1 cells24)) and IL-23 (which mediates IL-17 production in differentiated Th17 cells25)) were evaluated by qPCR. IL-12p35, IL-12p40, and IL-23p19 were measured because IL-12 and IL-23 share IL-12p40 as a co-subunit. The expression levels of both IL-12p35 and IL-12p40 mRNA were significantly increased (5.1-fold and 13.8-fold, respectively), while IL-23p19 mRNA expression remained at the same level (Figs. 4E–G) in the colonic tissue of IL-10−/− mice compared with wild-type littermates. However, expression levels of all three mRNAs were significantly decreased in the IM group compared with the control group (Figs. 4E–G, respectively). It was notable that the level of IL-23p19 mRNA was significantly decreased in the IM group even when compared with that of wild-type littermates. These results suggest that suppression of IFN-γ and IL-17 by IM may be due to the decreased levels of IL-12 and IL-23.
IM Treatment Significantly Suppressed IL-12/23 Gene Expression and Protein Secretion in Mouse Monocyte/Macrophage CellsTo investigate the mechanism underlying the suppression of IL-12p35, IL-12p40, and IL-23p19 mRNA in the colonic tissues of IL-10−/− mice treated with IM, we examined the effects of IM on proinflammatory cytokine expression in monocyte/macrophage cells in vitro. We chose J774A.1 monocyte/macrophage cells in this study because they have been reported to produce not only IL-12p40 but also TNF-α and IL-1β in a dose-dependent manner when stimulated by LPS.21) In this study, the cells were incubated with various concentrations of IM for 24 h. TNF-α mRNA expression was significantly decreased at all IM concentrations tested, with a decrease of as much as 20%. IL-1β and IFN-γ mRNA levels were not altered (Figs. 5A–C). Intriguingly, the levels of both IL-12p40 mRNA and IL-23p19 mRNA were decreased dose-dependently, and IL-23p19 expression was significantly suppressed at 10−4 M IM (69.7±4.6%), while IL-12p40 expression suppression was not statistically significant (55.4±19.4%) (Figs. 5D, E). These results prompted us to investigate the effects of IM on IL-12p40 and IL-23 protein secretion levels from these mouse monocyte/macrophage cells. ELISA using the cell culture medium revealed dose-dependent and significant decreases in the secretion of both IL-12p40 and IL-23. It was notable that IL-23 secretion was markedly suppressed to below the level of detection by IM at a concentration of 10−4 M (Figs. 5F, G). These in vitro studies suggest that the mechanisms by which IM treatment ameliorates colitis in IL-10−/− mice involve strong suppressive effects on the expression of IL-12 and IL-23 in monocyte/macrophage cells.
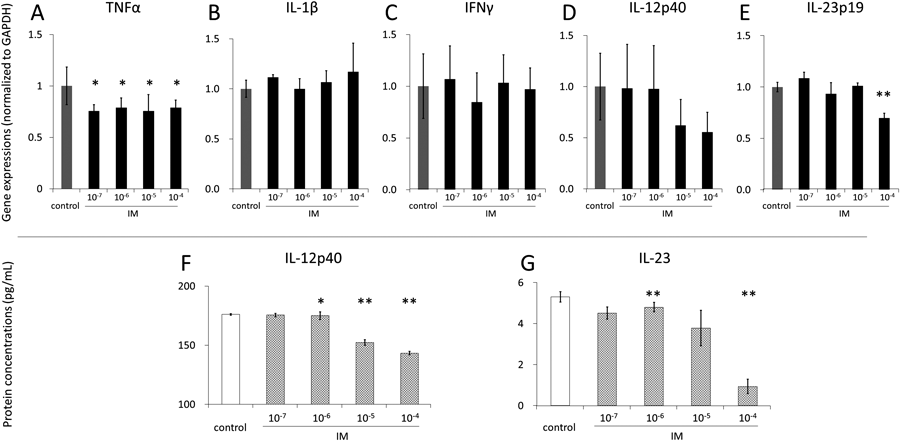
Graphs in A–E show control cell treated with 100% ethanol as gray bars and cells treated with IM (concentration range: 10−7–10−4 M) as black bars. The scales on the left side of the graphs indicate relative quantification compared with gene expression of controls. Graphs F and G show control cells treated with 100% ethanol as white bars and the cells treated with IM (concentration range: 10−7–10−4 M) as crosshatched bars. Data are expressed as mean±S.E.M. Dunnett’s test was used for statistical analysis. * p<0.05, ** p<0.01 compared with controls.
This study demonstrated that oral administration of irsogladine maleate (IM), an anti-peptic ulcer drug approved for clinical use, has prophylactic effects on spontaneous colitis in the colon of IL-10−/− mice. The mechanism appears to involve suppression of IL-12 and IL-23 production, which in turn regulates the Th1/Th17 immune response. Oral treatment with IM prevented colitis in IL-10−/− mice both macroscopically and histologically, and markedly reduced expression levels of proinflammatory cytokine genes, such as TNF-α and IL-1β, in the colonic mucosa. Significant decreases of IFN-γ and IL-17 expression levels in the IM group suggested that both Th1 and Th17 axes were suppressed by treatment with IM, and significant decreases in expression levels of IL-12p35, IL-12p40, and IL-23p19 in the colonic tissues suggested that both Th1 and Th17 axes were suppressed upstream. The results of in vitro experiments showed a direct effect of IM on reducing both gene expression and protein secretion of IL-12p40 and IL-23p19 in monocyte/macrophage cells, suggesting that the anti-inflammatory effects of oral IM treatment in vivo may be due to the direct effects of IM on monocyte/macrophage cells in the colonic mucosa.
Since 1984, various mechanisms of action of IM to protect the mucosa in the gastrointestinal tract were reported,14) including a few articles referring to anti-inflammatory effects in colitis. Among these reports, Yamaguchi et al. showed that IM prevented fibrosis in a dioctyl sodium sulfosuccinate (DSS)-induced colitis model through suppressing the production of proinflammatory cytokines,15) and Kamei et al. reported that one of the mechanisms underlying amelioration of experimental colitis by IM may be correlated with secretion of mucus preventing bacterial invasion by disrupting mucosal integrity induced with indomethacin.16) However, direct effects of IM in regulating the immune system were not reported except in this study. It is well known that the development of spontaneous colitis in IL-10−/− mice involves the interaction with resident enteric bacteria and local macrophages,26) and IL-10 produced by antigen-presenting cells is a key cytokine for maintenance of the homeostatic T-cell response to commensal bacteria.27) We demonstrated that IM suppressed secretion of IL-12 and 23 from monocyte/macrophage cells in vitro, and so the main effect of IM in preventing spontaneous colitis of IL-10−/− mice may involve suppression of secretion of upstream cytokines in the Th1 and Th17 axis by resident macrophages via regulation of the IL-12/23 response.
Recent studies regarding the pathogenesis of IBD indicated that the major proinflammatory cytokines, such as IFN-γ and IL-17, are produced by differentiation of Th1 and Th17 cells in Crohn’s disease.5,28) To explain the interaction of Th17 with the Th1 immune response, Fuss and Strober proposed that the Th1 response was predominant mainly in the initial most intense phase of colitis and a mixed Th1/Th17 response occurred in the later phase based on findings in murine models of colitis.28) The results of our in vivo study suggested that oral administration of IM may be effective in both the initial inflammatory phase and in the later phase of colitis, because oral administration of IM was shown to inhibit both Th1 and Th17 pathways.
Our in vitro study demonstrated direct and clear effects of IM acting to suppress IL-12p40 and IL-23p19 gene expression and protein secretion in monocyte/macrophage cells. Notably, the expression of IL-12p40 mRNA was decreased to below the limit of detection when the cells were treated with IM. One possible mechanism by which IM decreased mRNA expression involves epigenetic effects on histone deacetylase 3 (HDAC3), because IL-10 has been shown to suppress IL-12/23 production through histone deacetylation.29) We speculate that the main efficacy of IM in amelioration of colitis was to suppress IL-12p40 in intestinal monocyte/macrophage cells because IL-12p40 is a subunit common to both IL-12 and IL-23. IL-12 is involved in the differentiation of naive T cells into Th1 cells and IL-23 promotes expansion and maintenance of Th17 cells, both of which have been implicated in the pathogenesis of chronic inflammation.30,31) Taken together, the results of this study indicated that oral administration of IM inhibits the mucosal IL-12/IFN-γ Th1 pathway and the IL-23/IL-17 Th17 pathway, leading to amelioration of colonic inflammation. We propose that oral administration of IM, a well-tolerated drug, would be effective in ameliorating mucosal Th1/Th17-dominant immune responses, such as that in Crohn’s disease.
Several agents other than IM have been reported to ameliorate spontaneous colitis in IL-10−/− mice. Fenofibrate, a peroxisome proliferator-activated receptor α (PPARα) inhibitor, suppressed IFN-γ and IL-17 mRNA expression by splenocytes and isolated T cells from these mice and improved the colitis, indicating that fenofibrate and IM exert similar effects on T cells.32) Histidine, one of the constituent amino acids of an elemental diet, improved colitis and inhibited lipopolysaccharide (LPS)-induced nuclear factor-kappa B (NF-κB) in macrophages.33) Another clinical research focus regarding Crohn’s disease is to identify means of suppressing IL-12/23 production. Ustekinumab, an anti-human IL-12/23 monoclonal antibody, increased response rates in patients with Crohn’s disease that were resistant to anti-TNF-α therapy.34) Other results suggest that IL-23 may play a critical role in regulating the differential Th1/Th17 balance in both ulcerative colitis and Crohn’s disease,35) and thus IM may contribute to the development of new strategies for the treatment of patients with IBD.
This study had some limitations. Although we demonstrated a strong prophylactic effect of IM against spontaneous colitis in IL-10−/− mice, we did not investigate therapeutic effects of IM when colitis had already developed. In addition, we investigated the efficacy of IM in a mouse model of colitis and in mouse monocyte/macrophage cell lines. Therefore, prospective clinical studies to verify the efficacy of oral administration of IM in patients with IBD are required. IM has been approved for several decades for use in treating peptic ulcers in Japan at a dose of 4 mg per day, and is recognized as a well-tolerated drug.
In conclusion, our data indicated that oral IM exerts prophylactic effects on spontaneous colitis in the colon of IL-10−/− mice through regulation of the Th1/Th17 immune response by suppressing IL-12 and IL-23 production. This study suggested that oral administration of IM, a well-tolerated drug, may be a novel strategy for the treatment of IBD.
We are grateful to Fumie Saegusa for excellent technical assistance.
This study was supported in part by a Grant from Nippon-Shinyaku Co., Ltd. (Kyoto, Japan).