2015 年 38 巻 11 号 p. 1801-1808
2015 年 38 巻 11 号 p. 1801-1808
Atrial natriuretic peptide (ANP) plays an important role in vascular functions such as blood pressure regulation and relaxant activity. Individual vascular beds exhibit differences in vascular reactivity to various ligands, however, the difference in responsiveness to ANP between carotid and renal arteries and the molecular mechanisms of its vasorelaxant activity in a pathophysiological state, including hypertension, remain unclear. We therefore investigated this issue by exposing carotid and renal artery rings obtained from spontaneously hypertensive rats (SHR) to ANP. In the SHR artery (vs. control WKY artery), the ANP-induced relaxations were reduced in carotid artery but not renal artery. Acetylcholine-induced relaxations were reduced in both arteries in SHR (vs. WKY). Sodium nitroprusside-induced relaxation was similar in both arteries between the groups. In carotid arteries, the ANP-induced relaxation was not affected by endothelial denudation or by treatment with inhibitors of nitric oxide synthase, cyclooxygenase, the voltage-dependent potassium channel, or ATP-sensitive potassium channel in arteries from both SHR and WKY. In the carotid artery from WKY but not SHR, the ANP-induced relaxation was significantly reduced by inhibition of the large-conductance calcium-activated potassium channel (BKCa). The BKCa activator-induced relaxation was reduced in the SHR artery (vs. WKY). These results suggest that ANP-induced relaxation is impaired in the carotid artery from SHR and this impairment may be at least in part due to the reduction of BKCa activity rather than endothelial components.
Hypertension contributes markedly to global cardiovascular morbidity and mortality.1–3) Vascular dysfunction is a hallmark of hypertension and frequently leads to the development of atherosclerosis, stroke, and peripheral arterial disease.1,4) In vascular dysfunction, abnormalities of vascular smooth muscle cells and endothelial cells are key factors in the development of hypertension-associated vascular complications.5–7) It has been shown that endogenous vasoactive peptides such as angiotensin II, endothelin-1, urotensin II, and natriuretic peptides play an important role in the regulation of vascular tone in (patho)physiological conditions.8–11) Several reports suggested that the signalings of vasoactive peptides, including their production, metabolism, and affected vascular functions, are altered in arteries from hypertensive patients and animal models.6,10,12) Therefore, the manipulation of vasoactive peptide signaling is one of the most important strategies against the development of vascular dysfunction in hypertension.
Atrial natriuretic peptide (ANP) has received particular attention since its effects on the cardiac function and blood pressure regulation have been reported through various mechanisms, including diuretic, natriuretic, and vasorelaxation.13–21) Moreover, ANP could regulate vascular remodeling and exert anti-inflammatory and anti-fibrotic effects.15) The marked utility of circulating levels of ANP as biomarkers for various cardiovascular diseases, including hypertension, is becoming an emerging clinical issue.15,22,23) Plasma levels of ANP are increased under pathological conditions, such as myocardial infarction, pulmonary hypertension, left ventricular hypertrophy, heart failure, and hypertension.23–25) These increased ANP levels, by endogeneous production or exogenous administration could enhance natriuresis and diuresis, and lead to vascular relaxation, thereby reducing the blood pressure and circulatory volume, and, therefore, the cardiac workload.18) Although the regulation of ANP signaling is a potential therapeutic target against the development of hypertension-associated complications, the understanding of its signaling in hypertensive arteries remains unclear.
There is a growing body of evidence suggesting mechanisms underlying ANP-induced relaxation in arteries.26–31) In general, ANP leads to vasorelaxation via the A-type natriuretic peptide receptor (NPR-A)/particulate (membrane-bound) guanylyl cyclase (GC)/ guanosine 3′,5′-cyclic monophosphate (cGMP) pathway.24,32,33) Endothelium-derived substances modulate ANP-induced relaxation; however, their regulatory role is complicated. For example, ANP-induced relaxation was reduced28) or enhanced34) by endothelium denudation. Liang et al.34) found that endothelium-derived nitric oxide (NO) suppresses ANP-induced relaxation in the porcine coronary artery via desensitizing the large-conductance calcium-activated potassium channel (BKCa). Moreover, Tanaka et al. found that ANP-induced relaxation was inhibited by iberiotoxin, a specific blocker of BKCa, in the mesenteric artery30,31) but not aorta31) isolated from the rat, and activation of the voltage-dependent K+ (Kv) channel partly contributed to ANP-induced relaxation in the rat aorta.31) Although the above evidence suggests that regional differences of ANP-induced arterial responses are present, how and to what extent various signaling cascades are utilized in ANP-induced relaxation in the renal and carotid arteries of hypertensive animals remain unclear.
The main aim of the present study was to investigate our basic hypothesis that the vasorelaxant effects of ANP in renal and carotid arteries are impaired in the spontaneously hypertensive rat (SHR),35) which is a widely used experimental genetic hypertensive model in the study of hypertension-associated vascular dysfunction.36–39) To test our hypothesis, we performed vascular functional studies as well as experiments to identify the molecular mechanisms involved.
ANP was purchased from LKT Laboratories, Inc. (St. Paul, MN, U.S.A.). Phenylephrine (PE), 4-aminopyridine (4-AP), sodium nitroprusside (SNP), NG-nitro-L-arginine (L-NNA), indomethacin, 1,3-dihydro-1-[2-hydroxy-5-(trifluoromethyl)phenyl]-5-(trifluoromethyl)-2H-benzimidazol-2-one (NS1619), glibenclamide, and monoclonal β-actin antibody were all purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.). Acetylcholine (ACh) chloride was purchased from Daiichi-Sankyo Pharmaceuticals (Tokyo, Japan). Iberiotoxin was purchased from Peptide Institute, Inc. (Osaka, Japan). Sildenafil was purchased from Tocris Bioscience (Ellisville, MO, U.S.A.). All concentrations are expressed as the final molar concentration of the base in the organ bath. Horseradish-peroxidase-linked secondary anti-mouse antibody was purchased from Promega (Madison, WI, U.S.A.). Primary antibody against the BKCaβ1 subunit was from Alomone Labs (Jerusalem, Israel), while an antibody against protein kinase G-1 (PKG-1) was obtained from Cell Signaling Technology (Danvers, MA, U.S.A.).
Animals and Experimental DesignMale SHR and control WKY were obtained at the age of 4 weeks (Hoshino Laboratory Animals, Inc., Ibaraki, Japan). All animals were allowed access to a standard laboratory diet and water ad libitum in a controlled environment (room temperature: 21–22°C, room humidity: 50±5%) until the rats were 14 or 15 weeks old. This study was approved by the Hoshi University Animal Care and Use Committee, and all studies were conducted in accordance with “Guide for the Care and Use of Laboratory Animals” published by the U.S. National Institutes of Health, and “Guide for the Care and Use of Laboratory Animals” adopted by the Committee on the Care and Use of Laboratory Animals of Hoshi University (which is accredited by the Ministry of Education, Culture, Sports, Science and Technology of Japan). Systolic blood pressure (SBP) was measured as described previously.40)
Vascular Functional StudyVascular isometric force was recorded as in our previous papers.41–44) For the relaxation studies, renal and carotid arterial rings were precontracted with a submaximal concentration of PE (i.e., 1–3 µM). When the PE-induced contraction had reached a plateau, ACh (10−9–10−5 M), ANP (10−10–10−7 M), or SNP (10−10–10−5 M) was added in a cumulative manner. After the addition of sufficient aliquots of the agonist to produce the chosen concentration, a plateau response was allowed to develop before the addition of the next concentration of the same agonist. In the second series of functional experiments in carotid arteries, concentration–response curves for ANP (10−12–10−7 M) were constructed in the absence and presence of various inhibitors, including the NO synthase (NOS) inhibitor L-NNA (10−4 M), the non-selective cyclooxygenase (COX) inhibitor indomethacin (10−5 M), the inhibitor of the Kv channel 4-AP (10−6 M), the inhibitor of ATP-sensitive K+ (KATP) channel glibenclamide (10−5 M), or the BKCa inhibitor iberiotoxin (10−7 M) in endothelium-intact or -denuded preparations. These concentrations of inhibitors were chosen based on previous studies. Endothelium denudation was carried out by infusing a 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate solution (CHAPS, 0.1%, for 1 min), which was subsequently flushed out with KHS and its inability of ACh to relax these segments confirmed the success of this procedure, as reported previously.42,43) We also performed experiments to construct a concentration-response curve for NS1619 (a BKCa activator, 10−7–3×10−5 M) or sildenafil [a phosphodiesterase 5 (PDE5) inhibitor, 10−10–10−5 M] in carotid artery.
Protein Expression of BKCa Subunits and PKG-1 by Western BlottingProtein levels of subunits of BKCa and PKG-1 were quantified using immunoblotting procedures, essentially as described previously.42,43) Carotid arterial protein extracts (40 µg) were applied to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride membranes. Blots were incubated with the anti-BKCaα subunit (ca. 125 kDa; 1 : 500), anti-BKCaβ1 subunit (ca. 28 kDa; 1 : 1000), anti-PKG-1 (ca. 78 kDa; 1 : 1000), or anti-β-actin (ca. 42 kDa; 1 : 5000) antibodies, with detection being achieved using a horseradish peroxidase-conjugated IgG followed by enhanced chemiluminescence. The band intensity was quantified using CS Analyzer 3.0 software (ATTO, Tokyo, Japan).
Data and Statistical AnalysisData are expressed as mean±standard error (S.E.). Relaxation responses are shown as a percentage of the PE-induced pre-contraction. For the functional studies, individual concentration–response curves were analyzed using a nonlinear regression curve fitting relaxation–drug concentration relationships to determine Emax (the maximal response generated by the relaxant drugs), pD2 (a negative logarithm of EC50 which is the concentration of the agonist producing 50% of Emax), and area under the curve (AUC), using Graph Pad Prism software (ver. 5.0 for Mac, San Diego, CA, U.S.A.). The pD2 value of ACh-induced relaxation was calculated from the concentration–response curve between 10−9 and 10−6 (for carotid artery) or 10−6.5 M (for renal artery) since the maximal response was seen at 10−6 M. Statistical analysis was carried out with either an unpaired Student’s t-test or one-way ANOVA followed by Dunnett’s post-hoc test for effects of inhibitors or endothelium denudation on ANP-induced relaxation in carotid arteries from SHR and WKY. * p<0.05 was considered significant.
As shown in Table 1, at the time of experiments, SHR displayed higher SBP (vs. control WKY). The body weight was not significantly changed between groups (Table 1). The left ventricle-to-body weight ratio, which is an index of left ventricular hypertrophy,45) was significantly higher in SHR than WKY (Table 1).
| SHR (28) | WKY (26) | |
|---|---|---|
| SBP (mmHg) | 188±3* | 110±3 |
| BW (g) | 357.5±4.4 | 367.1±7.0 |
| LV/BW ratio (mg/g) | 2.47±0.03* | 2.05±0.02 |
Values are mean±S.E. Number of determinations within parentheses. * Significant difference from WKY control (p<0.05 by Student’s unpaired t-test). SBP, systolic blood pressure (measurement at 13 weeks old); BW, body weight (measurement at sacrifice: 14 or 15 weeks old); LV, left ventricular (measurement at sacrifice: 14 or 15 weeks old).
When the PE-induced arterial contraction had reached a plateau, ANP, ACh, or SNP was added cumulatively in renal (Fig. 1) and carotid (Fig. 2) arteries. In renal arteries, the ANP-induced relaxation was similar between SHR and WKY groups (Fig. 1A), whereas ACh-induced endothelium-dependent relaxation was reduced in SHR group compared to WKY group (Fig. 1B). SNP-induced endothelium-independent relaxation was similar between two groups (Fig. 1C). In carotid arteries, the ANP-induced relaxation was significantly weaker in SHR than in WKY (Fig. 2A). The ACh-induced endothelium-dependent relaxation was also significantly weaker in carotid arteries from SHR compared to WKY (Fig. 2B), although the pD2 value was not significantly different between SHR (7.64±0.15, n=8) and WKY [7.64±0.13 (p>0.05), n=8]. On the other hand, SNP-induced endothelium-independent relaxation was not significantly different between SHR and WKY (Fig. 2C). These results suggest that regional difference of ANP-induced relaxation between renal and carotid arteries was present in SHR. Therefore we further employed the experiments for mechanisms underlying the impaired ANP-induced relaxation in carotid arteries.

Data are mean±S.E. from 6 experiments. * p<0.05 vs. WKY.

Data are mean±S.E. from 6–9 experiments. * p<0.05 vs. WKY.
Next, to investigate the possible roles played by the endothelium, NO, prostanoid, and potassium channels, we performed a series of experiments in which ANP was added cumulatively to rings precontracted by PE following endothelium denudation or in the presence of various inhibitors, including 10−4 M L-NNA, 10−5 M indomethacin, 10−6 M 4-AP, 10−5 M glibenclamide, and 10−7 M iberiotoxin (Fig. 3, Table 2). Neither endothelium denudation (Figs. 3A, D, Table 2) nor the NOS inhibitor L-NNA (Figs. 3B, E, Table 2) had significant effects on the ANP-induced relaxation (vs. that observed in inhibitor-untreated endothelium-intact control rings) in either group of rats. The COX inhibitor indomethacin had no significant effect on the subsequent ANP-induced relaxation (vs. that observed in inhibitor-untreated rings) in either group of rats (Table 2). Neither the Kv channel inhibitor 4-AP nor KATP channel inhibitor glibenclamide had a significant effect on the ANP-induced relaxation in either group of rats (Table 2). Interestingly, although the BKCa inhibitor iberiotoxin had no significant effect on ANP-induced relaxation in SHR (Fig. 3C, Table 2), the relaxant response to ANP was significantly inhibited by iberiotoxin in WKY (Fig. 3F, Table 2). These results suggest that the reduced BKCa activity was responsible for impairment of the ANP-induced relaxation in the SHR carotid artery.

Inhibitors were treated for 30 min before the precontraction of PE and were present thereafter. Details are described in Materials and Methods. Data are mean±S.E. from 6–8 experiments. For comparison, the concentration–response curves for ANP of SHR depicted in Fig. 3A are shown again in B and C and the concentration–response curves for ANP of WKY depicted in Fig. 3D are shown again in E and F.
| SHR | WKY | |||||
|---|---|---|---|---|---|---|
| n | pS2 | Emax | n | pD2 | Emax | |
| Control | 8 | 9.07±0.09 | 89.6±3.5# | 7 | 9.31±0.12 | 98.5±1.2 |
| EC- | 7 | 8.93±0.17 | 95.3±2.5 | 6 | 9.07±0.15 | 96.8±1.1 |
| L-NNA | 8 | 9.06±0.15 | 95.1±1.3 | 8 | 9.46±0.18 | 98.1±1.9 |
| Indomethacin | 7 | 9.29±0.08 | 97.4±2.0 | 8 | 9.46±0.19 | 96.0±3.4 |
| 4-AP | 8 | 9.06±0.12 | 96.2±2.0 | 8 | 9.35±0.19 | 95.2±3.0 |
| Glibenclamide | 7 | 9.00±0.12 | 93.1±2.9 | 7 | 9.33±0.16 | 90.9±5.7 |
| IbTX | 7 | 9.00±0.22 | 96.4±1.3 | 8 | 9.02±0.23 | 83.6±5.5* |
Values are mean±S.E.: n, no. of experiments. Shown are comparisons of the sensitivity (pD2) and Emax to ANP in the absence (Control) and presence of inhibitors or endothelium denudation (EC-). * Significant difference from the corresponding control ANP-induced response in carotid arteries within SHR/WKY (p<0.05 by Dunnett’s test): # Significant difference from WKY control (p<0.05 by Student’s unpaired t-test).
Previous reports suggested that BKCa plays an important role in ANP-induced relaxation,29–31) and the present data suggest that BKCa activity may be responsible for the reduced ANP-induced relaxation in the SHR carotid artery. To examine whether BKCa-related relaxation is altered in carotid arteries from SHR, we investigated the effect of an activator of BKCa on relaxation in carotid arteries (Fig. 4A). The BKCa activator NS1619 led to relaxation in the carotid artery, and this relaxation was significantly reduced in SHR (vs. WKY) (Fig. 4A).
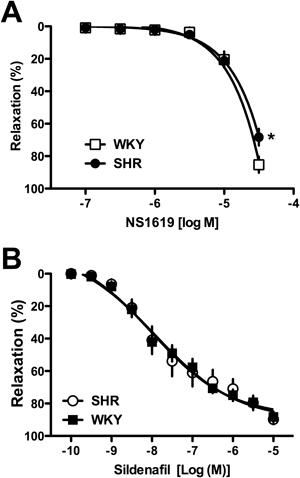
Data are mean±S.E. from 6–8 experiments. * p<0.05 vs. WKY.
In view of the published evidence that ANP-induced responses are mediated by the NPR-A/guanosine 5′-cyclic monophosphate (cGMP) pathway,24,32,33) we subsequently examined the effect of an inhibitor of cGMP-specific phosphodiesterase, PDE5,46,47) on relaxation in carotid arteries (Fig. 4B). The PDE5 inhibitor sildenafil led to relaxation in carotid arteries, and the response was not altered between SHR and WKY (Fig. 4B).
Expression of BKCa and PKG-1 in Carotid ArteryWe finally examined the expressions of BKCa subunits and PKG, a major down-stream pathway of cGMP,48,49) in carotid arteries by Western blotting analysis (Fig. 5). Neither the protein expression levels of the BKCaα subunit (Fig. 5A) nor β1 subunit (Fig. 5B) was altered between the two groups. Protein expression of PKG-1 in the carotid artery was similar between the two groups (Fig. 5C).

Analysis of protein expression for BKCaα (A), BKCaβ1 (B), and PKG-1 (C) in carotid arteries from SHR and WKY. Upper: Representative western blots of BKCa subunits, PKG-1, and β-actin. Lower: Bands were quantified as described in Materials and Methods. Data are mean±S.E. from 10 experiments.
ANP is a potent natriuretic and vasodilator peptide that is increased in patients with cardiovascular diseases, including heart failure, diabetes, and hypertension.21,23,26) Despite these marked (patho)physiological effects, little is known about the mechanisms by which ANP causes relaxation. Moreover, there is limited clear information on whether ANP-induced relaxations are impaired in hypertensive models. The present study revealed the impairment of ANP-induced relaxation in carotid but not renal arteries from SHR, and this impairment in the carotid artery of hypertensive rats may be at least in part due to the reduction of BKCa activity rather than endothelial components.
There have been several controversial studies on the modulatory effect of the endothelium and role of endothelium-derived factors, such as NO and COX metabolites, on ANP-induced relaxation.26–28,34) For example, ANP-induced relaxation was reduced28) or increased34) by endothelium denudation. ANP-induced relaxation was impaired in renal arteries from the diabetic rabbit at least partly due to reduced modulation by prostacyclin.26) In the present study, ACh-induced endothelium-dependent relaxation was impaired in carotid arteries from SHR, whereas SNP-induced endothelium-independent relaxation was similar between SHR and WKY. These data suggest that the carotid artery of SHR at this age exhibits endothelial dysfunction. In such arteries, the ANP-induced relaxations were not affected by endothelium denudation or NOS and/or COX inhibition in either SHR or WKY carotid arteries. Thus, the impairment of ANP-induced relaxation in the carotid artery of SHR is responsible for endothelium-independent mechanisms despite exhibiting endothelial dysfunction.
There is an emerging body of evidence suggesting that K+ channels play an important role in controlling the vascular tone and ANP-mediated responses. Tanaka et al. found that the ANP-induced relaxation was inhibited by iberiotoxin, a specific blocker of BKCa, in the rat mesenteric artery30,31) but not rat aorta,31) and activation of the Kv channel partly contributed to the ANP-induced relaxation in the rat aorta.31) Marrachelli et al.26) found that the impaired ANP-induced relaxation in the renal artery of diabetic rabbits was at least partly due to the impaired participation of KATP, Kv, and KCa channels. Recently, Stott et al.50) observed that the ANP-induced vasodilation was mediated by Kv7 channels in the rat aorta and renal artery, and suggested that the reduced ANP-induced vasodilation in such arteries of SHR was attributable to impaired Kv7 activity. In the present study, ANP-induced relaxation was altered by neither 4-AP (Kv inhibitior) nor glibenclamide (KATP inhibitor) in carotid arteries from both SHR and WKY. Notably, iberiotoxin (BKCa inhibitor) reduced the ANP-induced relaxation in carotid arteries from WKY but not SHR. These results suggest that the contribution of BKCa activity to modulate ANP-induced relaxation is lacking in the SHR carotid artery. Further support for this hypothesis is provided by the observations that the BKCa activator NS1619 led to reduced relaxation in carotid arteries from SHR compared to WKY.
BKCa are expressed largely in vascular smooth muscle cells, where they function as negative-feedback regulators of the vascular tone.51–53) BKCa are composed of pore-forming α and regulatory β1 subunits.51–54) The alteration of BKCa subunit expression and function occurs in vascular smooth muscle from hypertensive models.52,55) In the present study, marked changes of BKCa subunits were not detected in carotid arteries between SHR and WKY at this age. Because the activity of BKCa is regulated not only by the expression of its subunits, but also by various messengers including calcium, heterotrimeric GTP-binding proteins, reactive oxygen species, protein phosphatase, and protein kinases,52,53,56) these pathways may modulate BKCa activity in hypertensive carotid arteries. An increased cGMP/PKG pathway leads to BKCa activation. In the present study, we found that carotid arterial relaxation induced by sildenafil, an inhibitor of PDE5, was similar between SHR and WKY, and that the protein expression of PKG-1 in the carotid artery was similar between the two groups. These data suggest that the reductions of ANP-induced relaxation and BKCa activity were not responsible for cGMP/PKG. Further studies will be required to determine the molecular mechanisms of BKCa activation induced by ANP in carotid arteries.
In conclusion, the present study provides evidence that reduced BKCa activity may make particularly important contributions to the carotid arterial hyporeactivity to ANP displayed by SHR. As abnormal vascular smooth muscle responses may contribute to the etiology of hypertension-associated vascular complications, our study raises the possibility that manipulations of ANP signaling, such as by the activation of vascular BKCa activity, may help prevent vascular complications developing in the presence of hypertension.
We thank R. Arai, C. Uehara, C. Kanazu, N. Sagawa, M. Toba, T. Adachi, M. Oda, K. Matsubara, Y. Noishiki, J. Takagi, Y. Kimoto, H. Higa, and M. Nagata for technical assistance. This study was supported in part by JSPS KAKENHI Grant Numbers 26460107, 15K21419, and 15K07975 and by the Science Research Promotion Fund from the Promotion and Mutual Aid Corporation for Private Schools of Japan.
The authors declare no conflict of interest.