2015 年 38 巻 8 号 p. 1208-1213
2015 年 38 巻 8 号 p. 1208-1213
We demonstrated the inhibitory effect of fluvoxamine, a selective serotonin reuptake inhibitor (SSRI), on voltage-dependent K+ (Kv) channels in freshly isolated rabbit coronary arterial smooth muscle cells using a whole-cell patch clamp technique. Fluvoxamine reduced the amplitude of Kv currents in a concentration-dependent manner with an IC50 value of 3.71±1.09 µM and a Hill coefficient of 0.62±0.14. Although fluvoxamine did not significantly affect the steady-state activation curve, it shifted the steady-state inactivation curve toward a more negative potential. Pretreatment with another SSRI, paroxetine, did not affect the basal Kv current and did not alter the inhibitory effect of fluvoxamine on Kv channels. We concluded that fluvoxamine inhibits the Kv current in a concentration-dependent manner and in a closed (inactivated) state of the Kv channels independent of serotonin reuptake inhibition.
Fluvoxamine is a selective serotonin reuptake inhibitor (SSRI) that has been used for the treatment of major depression, anxiety, and panic disorder since 1983 in many countries, including those in Europe and Japan.1,2) The antidepressant activity of fluvoxamine is derived from inhibition of serotonin reuptake in the central nervous system with negligible effects on other neurotransmitter reuptake systems.3,4) A recent study indicated that fluvoxamine showed high affinity for the σ1 receptor, which has a neuromodulatory role on the neurotransmitter system and an antidepressant-like effect.5–7) Although fluvoxamine has beneficial effects in the treatment of depression, its administration is associated with common side effects, including nausea, anorexia, palpitations, headache, and dizziness.8) Fluvoxamine also affects ion channels, such as the human ether-a-go-go related (hERG) K+ and Kv1.5 channels.9,10) However, these studies were performed primarily in cultured cell lines rather than in native cells. Therefore, questions remain about the effect of fluvoxamine on ion channels from freshly isolated cells, particularly vascular smooth muscle cells.
Four types of K+ channel are expressed in vascular smooth muscle cells: voltage-dependent K+ (Kv), ATP-sensitive K+ (KATP), inward rectifier K+ (Kir), and big-conductance Ca2+-activated K+ (BKCa) channels.11,12) Our previous reports suggested that Kv channels participate in the regulation of resting membrane potential and thus resting tone in some arteries.13,14) In addition, pathological states such as hypertension, diabetes, and hypoxia, are closely related with alteration of Kv channel activity and expression in the vasculature.15) Therefore, the adverse effects of drugs on Kv channels should be clearly determined to correctly interpret experimental data regarding vascular disorders.
Considering the usefulness of fluvoxamine as a SSRI and the physiological importance of Kv channels, the effects of fluvoxamine on vascular Kv channel should be clearly examined to avoid toxic effect on the vasculature when applying fluvoxamine as antidepressant drug.
In the present study, we investigated the effects of fluvoxamine on Kv channels using freshly isolated coronary vascular smooth muscle cells. Our results indicated that fluvoxamine inhibited vascular Kv channels in a concentration-dependent manner independent of serotonin reuptake inhibition.
New Zealand White rabbits of male (2.0–2.5 kg) were sacrificed by simultaneous injection of sodium pentobarbital (50 mg/kg) and heparin (100 U/kg) into the ear vein. The procedure was performed in accordance with the guidelines of the Committee for Animal Experiments of Kangwon National University. The hearts were rapidly removed, and the left descending coronary arteries were dissected in normal Tyrode’s solution. Single smooth muscle cells were obtained using a two-step enzyme treatment. First, the arteries were transferred to Ca2+-free normal Tyrode’s solution containing papain (1.0 mg/mL), bovine serum albumin (BSA, 1.5 mg/mL), and dithiothreitol (DTT, 1.5 mg/mL) and incubated for 25 min at 37°C. The solution was then replaced with Ca2+-free normal Tyrode’s solution containing collagenase (2.8 mg/mL), BSA, and DTT and incubated for 26 min at 37°C. The digested tissues were triturated by pasture pipette in Kraft–Brühe (KB) solution. The isolated cells were kept at 4°C and used on the day of preparation.
Solutions and ChemicalsThe normal Tyrode’s solution contained (mM): sodium chloride (NaCl), 135; NaH2PO4, 0.33; potassium chloride (KCl), 5.4; N-(2-hydroxyethyl)piperazine-N′-2-ethanesulfonic acid (HEPES), 5; CaCl2, 1.8; MgCl2, 0.5; glucose, 16.6; adjusted to pH 7.4 with NaOH. The KB solution contained (mM): potassium hydroxide (KOH), 70; KCl, 55; L-glutamate, 50; KH2PO4, 20; taurine, 20; MgCl2, 3; glucose, 20; HEPES, 10; ethyl glycol bis(2-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), 0.5; adjusted to pH 7.3 with KOH. The internal (pipette-filling) solution for the recording of Kv channels contained (mM): K-aspartate, 110; KCl, 25; HEPES, 10; Mg-ATP, 4; NaCl, 5; MgCl2, 1; EGTA, 10; adjusted to pH 7.2 with KOH. The activation of KATP and BKCa channels were excluded by inclusion of 4 mM ATP and 10 mM HEPES in the internal solution. Furthermore, the bath solution contained iberiotoxin (100 nM) to completely exclude the involvement of BKCa channels. Fluvoxamine was purchased from Sigma Chemical Co. (St. Louis, MO, U.S.A.) and dissolved in ethanol. Paroxetine was purchased from Tocris Cookson (Ellisville, MO, U.S.A.) and dissolved in dimethyl sulfoxide (DMSO).
ElectrophysiologyWhole cell Kv currents were recorded using the whole-cell patch clamp technique with an EPC-8 amplifier (Medical System Corp., Darmstadt, Germany) and digital interface NI-DAQ-7 (National Instruments, Union, CA, U.S.A.). Patch pipettes were made from borosilicate capillaries (Clark Electromedical Instruments, Pangboune, U.K.) using a PP-830 puller (Narishige Scientific Instrument Laboratory, Tokyo, Japan). The resistance of patch pipettes was 2–3 MΩ. Current signals were filtered at 0.5–1.0 kHz and sampled at 1–3 kHz.
Data AnalysisData analysis was performed using Origin 7.5 software (Microcal Software, Inc., Northampton, MA, U.S.A.). A first-order blocking scheme was applied to describe the kinetics of drug-channel interaction.13,16) The IC50 and Hill coefficient (n) were obtained by fitting concentration-dependence data (Fig. 2) using the Hill equation:
 |
The data for activation curves were derived from tail currents elicited by returning to −40 mV after short depolarizing pulses between −80 and +60 mV. The Boltzmann equation was used to fit the activation curves:
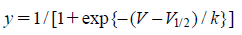 |
The steady-state inactivation data were obtained using a two-pulse voltage protocol; 7-s preconditioning pulses applied at potentials ranging from −80 to +30 mV in the absence and presence of drugs, then returned to +40 mV. Steady-state inactivation curves were fitted with another Boltzmann equation as follows:
 |
The results are presented as means±standard error of the mean (S.E.M.). Student’s t-test was used to estimate statistical significance and a value of p<0.05 was considered to indicate statistical significance.
The effects of fluvoxamine on Kv currents were measured in freshly isolated coronary arterial smooth muscle cells. To record the Kv current effectively, activation of BKCa and KATP channels was excluded by addition of EGTA and ATP in the pipette solution. Activation of Kir channels was also excluded by using coronary smooth muscle cells isolated from the second branch of the artery, known as the conduit artery, as the Kir channel has only been identified in small-diameter arteries.17,18) Figure 1A shows representative Kv currents in coronary arterial smooth muscle cells. The Kv current rapidly reached a peak and showed slow and partial intrinsic inactivation. Application of 3 µM fluvoxamine decreased the Kv current within 2 min (Fig. 1B). This inhibition was partially washed out (ca. 50%). The current–voltage (I–V) relationship showed that application of 3 µM fluvoxamine reduced the Kv currents by 48.63±1.53% at +60 mV (Fig. 1C).

Current traces were elicited by step depolarizing pulses between −60 and +60 mV with a holding potential of −60 mV in increases of 10-mV under control conditions (A) and in the presence of 3 µM fluvoxamine (B). (C) Current–voltage (I–V) relationship at steady-state Kv current under control conditions (○) and in the presence of 3 µM fluvoxamine (●). n=7. * p<0.05.
To determine whether the inhibition of Kv channels by fluvoxamine was dose-dependent, we applied various concentrations (0, 0.1, 0.3, 1, 3, 10, and 30 µM) of fluvoxamine to the Kv channels. The superimposed currents were elicited by applying a +60-mV depolarizing pulse from a holding potential of −60 mV. As shown in Fig. 2A, higher concentrations of fluvoxamine increased the inhibition of Kv current. The results of least-squares fitting using the Hill equation yielded an IC50 value of 3.71±1.09 µM and a Hill coefficient of 0.62±0.14 for steady-state inhibition (Fig. 2B). These results suggest that fluvoxamine dose-dependently inhibits Kv channels.

(A) Representative current traces were obtained in the presence of 0, 0.1, 0.3, 1, 3, 10, and 30 µM fluvoxamine. Each trace was elicited by 600-ms depolarizing pulse of +60 mV from a holding potential of −60 mV. (B) The concentration-dependent curve for fluvoxamine was measured at steady-state. n=9.
We investigated the effects of fluvoxamine on the activation and inactivation kinetics to confirm whether fluvoxamine shifted the activation and/or inactivation curves of the Kv channels. The activation curve was obtained from the tail current using the two-pulse protocol and was fitted with the Boltzmann equation. As shown in Fig. 3A, application of 3 µM fluvoxamine induced a slight right shift in the activation curve, that was not significant. The half-maximal activation potential (V1/2) and slope value (k) were −13.50±0.85 mV and 10.34±0.57 under control conditions, and −11.78±0.78 mV and 10.34±0.52 in the presence of 3 µM fluvoxamine, respectively.

(A) Activation curves in the absence (○) and presence of 3 µM fluvoxamine (●). Tail currents were elicited by returning to a potential of −40 mV after short depolarizing pulses (30–100 ms) between −80 mV and +60 mV in increments of 10-mV. The current data were normalized from the peak value of the second pulse (tail current), which was considered as channel activation. n=6. (B) Steady-state inactivation curves were calculated under control conditions (○) and in the presence of 3 µM fluvoxamine (●). The currents were recorded by a test pulse to +40 mV after the 7-s pre-pulses at various voltages between −80 and +30 mV, and the steady-state currents of the test pulse were normalized to the peak amplitude of the pre-pulses. The summarized data were fitted with the Boltzmann equation. n=7. * p<0.05.
The steady-state inactivation kinetics was obtained from the conventional double-pulse protocols, which were fitted with another Boltzmann equation. As shown in Fig. 3B, 3 µM fluvoxamine induced a significant leftward shift in the steady-state inactivation curve of Kv channels. The half-maximal potential (V1/2) and the slope value (k) were −33.86±0.86 mV and 8.15±0.59 under control conditions, and −41.32±1.04 mV and 7.55±0.46 in the presence of 3 µM fluvoxamine, respectively. These results suggest that fluvoxamine alters the voltage sensitivity of Kv channels and interacts with the Kv channel in the closed state.
Inhibitory Effect of Fluvoxamine in the Presence of Another SSRITo confirm that the inhibitory effect of fluvoxamine on Kv channels was not due to inhibition of serotonin reuptake, we recorded the effects of fluvoxamine on Kv channels in the presence of another SSRI, paroxetine. Pretreatment with 1 µM paroxetine did not affect the basal Kv current. Furthermore, the inhibitory effect of fluvoxamine was not altered by paroxetine (Figs. 4A, B). These results clearly suggest that the inhibitory effect of fluvoxamine is completely independent of serotonin reuptake inhibition.

(A) Currents were obtained by application of a one-step depolarizing pulses to +60 mV at a holding potential of −60 mV. Superimposed current traces of Kv current in the absence (control), in the presence of 1 µM paroxetine, and with the additional application of 3 µM fluvoxamine. (B) The results are summarized in panel A. n=6. * p<0.05. NS=not significant.
The present study revealed the effects of fluvoxamine on Kv currents in rabbit coronary arterial smooth muscle cells. Fluvoxamine inhibited the Kv current in a concentration-dependent manner independent of serotonin reuptake inhibition. Furthermore, fluvoxamine shifted the steady-state inactivation current curves of Kv channels toward a more negative potential, which suggested that fluvoxamine inhibited the Kv channel in a closed state.
Several lines of evidence support the suggestion that fluvoxamine inhibits Kv channels regardless of its own target. First, another SSRI, paroxetine, did not affect the basal Kv current and did not alter the inhibitory effect of fluvoxamine (Fig. 4). Second, the IC50 value of fluvoxamine on the Kv current was 3.71 µM (Fig. 2). This value was much larger than the reference IC50 value of 3.8 nM for serotonin reuptake inhibition,19) which suggests that the inhibitory effect of fluvoxamine on Kv channels was not due to inhibition of serotonin reuptake. Third, fluvoxamine shifted the steady-state inactivation curves of Kv channels toward a more negative potential (Fig. 3). This result suggested that fluvoxamine should change the voltage sensitivity of the Kv channels. Therefore, we concluded that fluvoxamine interacts with the Kv channel in the closed state. Fourth, the fluvoxamine-induced inhibition of Kv current occurred within 2 min, which supported a rapid interaction between the drug and the channel. Fifth, serotonin acts as vasoconstrictor and was reported to inhibit Kv channels in vascular smooth muscle.20) Although SSRIs may increase the serotonin concentration in the circulation, thereby increasing cardiovascular risk in specific hypertensive patients,21) this increase in serotonin by SSRIs was not possible in a single cell experimental system. Therefore, the inhibition of Kv current by fluvoxamine was not due to SSRI-induced increases in serotonin, but could be attributed to an interaction with Kv channels.
SSRIs such as fluoxetine, citalopram, escitalopram, dapoxetine, paroxetine, sertraline, and fluvoxamine, are widely used in the treatment of major depression, anxiety, and panic disorder.5) Their therapeutic effect is derived from their ability to block the reuptake of serotonin, while they exert minimal effects on other neurotransmitter systems.3,4) Fluvoxamine, as a representative SSRIs, has been widely and effectively used for treating depression.8) Although most SSRIs have no life-threatening side effects,22,23) additional side effects of fluvoxamine on K+ channels have been reported. For example, fluvoxamine inhibited the Kir4.1 channel in human embryonic kidney (HEK) 293T cells24) and G protein-activated inwardly rectifying K+ (GIRK) in Xenopus oocytes.25) Fluvoxamine also directly blocked the Kv1.5 current in Chinese hamster ovary (CHO) cells10) and the human ether-a-go-go related (HERG) K+ channel in HEK 293 cells.9) However, these studies were restricted to cultured cell lines and no studies have addressed to the effects of fluvoxamine on Kv channels in native vascular smooth muscle cells.
Vascular Kv channels have been regarded as one of the major channels involved in regulating the resting membrane potential and basal tone in vascular smooth muscle.11) Therefore, the side effects of some drugs on Kv channels should be clarified. Several research groups, including ours, have attempted to identify the side effects of chemicals that affect vascular Kv channels besides their own function. For example, curcumin (spice), the phosphoinositide-3 kinase inhibitor LY294002, the L-type Ca2+ channels inhibitor verapamil, the T-type Ca2+ channels inhibitor mibefradil, the protein kinase C inhibitor bisindolylmaleimide (I), and the guanylyl cyclase activator YC-1 directly inhibited vascular Kv channels in the open state.26–31) In addition, the Ca2+ channels inhibitor efonidipine and NNC 55-0396, as well as the tyrosine kinase inhibitor genistein and the protein kinase A inhibitor H-89 directly inhibited the vascular Kv channel in the closed state.13,14,32,33) Furthermore, our group also reported that the protein kinase C inhibitor staurosporine and the calmodulin inhibitor trifluoperazine inhibited the vascular Kv channel in both open and closed states.34,35) In the present study, we clearly demonstrated that fluvoxamine inhibited the Kv channel in the closed state. Therefore, this side effect on vascular Kv channels should be taken into consideration when using fluvoxamine as an antidepressant drug.
In our experiments, the IC50 value for fluvoxamine-induced inhibition of Kv channels was 1000 fold higher than the known IC50 value for serotonin reuptake inhibition (3.71 µM vs. 3.8 nM, respectively). It is important to note that the reported therapeutic plasma concentration of fluvoxamine is <0.8 µM when used in the treatment of depression in humans.10,36–38) While our reported fluvoxamine IC50 value for the inhibition of Kv channels was higher than the reported therapeutic plasma concentration, significant Kv channel inhibition was observed at lower concentrations of 0.3 and 1 µM (Fig. 2). Kv channel inhibition by fluvoxamine may therefore need to be taken into consideration when treating depression as there is the potential for several detrimental clinical side effects such as hypertension, diabetes, and hypoxia.
The physiological and pharmacological properties of vascular Kv channels have been relatively well studied in both rats and mice. However, the molecular subtypes of Kv channels in the rabbit remain undefined. Therefore, we could not determine which Kv subtypes were expressed in rabbit coronary artery, and could not address which Kv subtypes interacted with fluvoxamine. However, previous studies identified Kv1.1, Kv1.2, Kv1.4, Kv1.5, Kv2.1, and Kv9.3 subtypes in vascular smooth muscle.15,39,40) As Kv1.5 subtype is expressed in most arterial smooth muscle and fluvoxamine directly inhibited Kv1.5 channels expressed in CHO cells,10) at least one of the main targets of fluvoxamine could be the Kv1.5 subtype, although other subtypes may also be affected. Further studies are necessary to determine which Kv subtypes interact with fluvoxamine.
We found that fluvoxamine inhibited vascular Kv channels in the closed state, and this inhibition occurred independent of the inhibition of serotonin reuptake. Considering the important physiological role of Kv channels and the efficacy of fluvoxamine in the treatment of depression, the effects of this drug on vascular K+ channels should be considered when used in patients with depression.
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2014-R1A1A4A01003840). This research was supported by the Programs of the NRF funded by the Ministry of Education, Science and Technology (2012-M3A9C7050184). This study was supported by 2012 Research Grant from Kangwon National University (C1009233-01-01).
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.