2016 Volume 39 Issue 10 Pages 1581-1587
2016 Volume 39 Issue 10 Pages 1581-1587
Long-term peritoneal dialysis (PD) frequently produces morphological and functional changes of the peritoneum, making continuation of PD difficult. Therefore, it is necessary to evaluate peritoneal injury at an early stage and develop appropriate therapies. The aims of the present study were to evaluate peritoneal injury at an early stage and assess a drug for prevention of peritoneal injury using our previously developed novel evaluation method. Peritoneal injury was induced in model animals by intraperitoneal injection of methylglyoxal (MGO) for 1 to 5 consecutive days or chlorhexidine digluconate (CG) for 1 to 14 consecutive days. Tetramethylrhodamine-dextran (RD)-10 and fluorescein isothiocyanate-dextran (FD)-2000 were then injected into the peritoneal cavity and recovered after 120 min to evaluate peritoneal injury. The ratio of the concentration of RD-10 to FD-2000 (RD-10/FD-2000 ratio) significantly decreased in animals that had been treated with MGO or CG for 1 d. Moreover, the RD-10/FD-2000 ratio significantly increased in CG- and thalidomide-treated animals. The RD-10/FD-2000 ratio can be used to evaluate peritoneal injury at an early stage and assess the drug efficacy of thalidomide for prevention of peritoneal injury. This study will contribute to the development of therapeutic treatments for peritoneal injury.
Peritoneal dialysis (PD) is a major treatment for end-stage renal disease and a useful therapy to compensate for some cases of renal function decline. In particular, PD can preserve residual renal function and contribute to improving the quality of life in patients undergoing PD.1–3) In recent years, the “PD first,” strategy, which involves starting with PD and then switching to hemodialysis after utilizing residual renal function, has been anticipated to provide a good prognosis for patients. However, long-term PD frequently produces morphological and functional changes of the peritoneum, making continuation of PD difficult.4–8) Moreover, progression of peritoneal injury causes serious complications, such as encapsulating peritoneal sclerosis.9) Therefore, it is necessary to evaluate peritoneal injury at an early stage and develop appropriate treatments for peritoneal injury.
Some recent studies have shown that several drugs have preventive effects for peritoneal injury. Rosiglitazone, a peroxisome proliferator-activated receptor-γ (PPARγ) agonist, reduced fibrosis and angiogenesis in two studies.10,11) Renin–angiotensin–aldosterone system—blocking drugs such as the renin inhibitor aliskiren, angiotensin-converting enzyme inhibitors, and angiotensin II receptor blockers improved peritoneal morphology and function in other studies.12–14) Moreover, thalidomide prevented peritoneal fibrosis caused by chlorhexidine digluconate in rat and mouse experimental models.15,16) However, preparation of animal models of peritoneal injury requires a long duration of time and great effort. Moreover, evaluation methods of drug efficacy for peritoneal injury such as the peritoneal equilibration test (PET), polymerase chain reaction, Western blot analysis, and immunohistochemical staining are problematic. The PET, which is the most widespread method for evaluation of peritoneal function, requires a long period of time to complete. Polymerase chain reaction, Western blot analysis, and immunohistochemical staining are complicated and high-cost.
We have developed an evaluation method for peritoneal injury using dual macromolecular markers: tetramethylrhodamine-dextran (RD-10) and fluorescein isothiocyanate-dextran (FD-2000). Our evaluation method utilizes the ratio of the concentrations of these two markers (the RD-10/FD-2000 ratio).17) RD-10 in dialysate is suitable for evaluation of peritoneal injury, but the concentration of RD-10 is influenced by the transfer of water. FD-2000 compensates for the transfer of water. The RD-10/FD-2000 ratio can be used to easily evaluate peritoneal injury regardless of the drainage volume when the glucose concentration in the PD fluid (PDF) or the dosage volume has changed. When performing the PET, it is necessary to measure the concentrations of creatinine in the blood and PD effluent and glucose in the PD effluent. Because both creatinine and glucose are endogenous substances, the test data are influenced by the status of the patient. In contrast, our method uses exogenous substances; thus, the accuracy of our method may be superior to that of the PET. Moreover, the sensitivity of our method is high because of the measurement of fluorescence. Thus, we hypothesized that the RD-10/FD-2000 ratio can be used to evaluate peritoneal injury at an early stage.
In this study, we evaluated rat and mouse models of short-duration peritoneal injury. Mice are easier to handle because their body weight is approximately one-tenth that of rats. We assessed whether the RD-10/FD-2000 ratio can be utilized as the evaluation method for peritoneal injury at an early stage. Moreover, we evaluated the use of thalidomide for prevention of peritoneal injury by the RD-10/FD-2000 ratio.
Methylglyoxal (MGO) was obtained from Nacalai Tesque, Inc. (Kyoto, Japan). Chlorhexidine digluconate (CG) and FD were obtained from Sigma Chemical Co. (St. Louis, MO, U.S.A.). RD was obtained from Invitrogen (Carlsbad, CA, U.S.A.). Thalidomide and hematoxylin and eosin (H&E) were obtained from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Embedding medium for frozen tissue specimens (Tissue-Tek O.C.T. compound) was obtained from Sakura Finetek Japan Co., Ltd. (Tokyo, Japan). The PDF (Dianeal-N PD-4 1.5) was obtained from Baxter, Ltd. (Tokyo, Japan).
AnimalsMale Wistar rats (7 weeks old) and male ddY mice (5 weeks old) were housed in cages in an air-conditioned room and maintained on a standard laboratory diet (MF; Oriental Yeast Co., Ltd., Tokyo, Japan) and water ad libitum. All animal experiments in the present study conformed to the Guidelines for Animal Experimentation of Nagasaki University.
Preparation of Peritoneal Injury Animal ModelsRats were prepared for peritoneal injury by the following slightly modified protocol.18) Briefly, 20 mL of 20-mM MGO dissolved in saline was injected intraperitoneally for 1 to 5 consecutive days. In a control experiment, saline was intraperitoneally injected in the same fashion. Mice were prepared for peritoneal injury by intraperitoneal injection of 2 mL of 20- to 80-mM MGO. Rats were prepared for CG-induced peritoneal injury by the following slightly modified protocol.15) Briefly, saline containing 0.1% CG and 15% ethanol was injected intraperitoneally for 1 to 14 consecutive days at a volume of 10 mL/kg body weight. In a control experiment, saline containing 15% ethanol was intraperitoneally injected in the same fashion. Mice were prepared for CG-induced peritoneal injury by intraperitoneal injection of 0.01 to 0.1% CG at a volume of 10 mL/kg body weight.
Evaluation of Drug EfficacyPrevention of peritoneal injury was performed following a previously described protocol.15) Thalidomide (100 mg/kg) was suspended in 0.5 mL olive oil and orally administered using a gastric tube once daily after the first administration of CG. In a control experiment, olive oil was orally administered in the same fashion.
Assessment of Peritoneal Injury with the RD-10/FD-2000 RatioAssessment of peritoneal injury with the RD-10/FD-2000 ratio was performed following a previously described protocol.17) Rats or mice were anesthetized with sodium pentobarbital (60 mg/kg, intramuscular injection). The skin was cut and the abdominal wall exposed. FD-2000 (molecular weight (MW) 2000000, 0.05 mg, 5 µg/mL) and RD-10 (MW 10000, 0.1 mg, 10 µg/mL) dissolved in 10 mL of PDF (Dianeal-N PD-4 1.5 containing 1.36% glucose) were injected into the peritoneal cavity of the rats using a syringe with a 21-G×1.5-inch needle (Nipro Co., Ltd., Osaka, Japan). Alternatively, 1 mL of PDF was injected into the peritoneal cavity of the mice using a syringe with a 26-G×0.5-inch needle (Nipro Co., Ltd.). The needle was then removed, and the pinhole in the abdominal wall was closed with surgical adhesive (Aron Alpha A; Daiichi Sankyo Pharmaceutical Co., Ltd., Tokyo, Japan) to prevent leakage of the fluid. The entire residual solution in peritoneal cavity was recovered 120 min after the injection.
Quantification of MarkersThe obtained samples were centrifuged at 1600×g at 4°C for 15 min, and the supernatants were obtained. The quantification conditions were as follows.
For quantification of FD-2000, the sample (20 µL) was diluted with 500 µL of phosphate-buffered saline (PBS) and then measured using a spectrofluorophotometer (RF-1500; Shimadzu Corp., Kyoto, Japan) at excitation and emission wavelengths of 494 and 521 nm, respectively. For quantification of RD-10, the sample (40 µL) was diluted with 500 µL of phosphate-buffered saline and then measured using the same spectrofluorophotometer at excitation and emission wavelengths of 555 and 580 nm, respectively.
Histological ExaminationHistological analysis was performed by H&E staining. After recovery of the residual solution in the peritoneal cavity, the abdominal walls were cut and fixed with 4% paraformaldehyde in phosphate buffer. They were then embedded in O.C.T. compound frozen at −80°C, and sliced into 10-µm sections using a microtome (Retoratome REM-710; Yamato Kohki Industrial Co., Ltd., Saitama, Japan) and stained by H&E. Stained samples were observed using a microscope with a 10× objective lens.
Statistical AnalysesStatistical comparisons between two groups were performed by an unpaired Student’s t-test (Figs. 1, 3, 5a, 5c, 7, 8), and multiple comparisons were performed by two-way ANOVA followed by Dunnett’s test (Figs. 5b, d). A p value of <0.05 was considered to indicate statistical significance.
To evaluate peritoneal injury at an early stage using the RD-10/FD-2000 ratio, MGO (which is generally used for 5 consecutive days to induce peritoneal injury) or CG (which is generally used for 14 consecutive days to cause peritoneal injury) was administered to the rats for a short period. The RD-10/FD-2000 ratio significantly decreased in rats treated with MGO for 1 to 5 consecutive days compared with those in saline (vehicle)-treated rats (Fig. 1a). The recovery volume of peritoneal fluid 120 min after injection was also significantly decreased in MGO-treated rats (Fig. 1b). Figure 2 shows the peritoneum of vehicle-treated and MGO-treated rats by H&E staining. The peritoneum of rats treated with MGO for 1 d did not exhibit an altered morphology, but that in rats treated with MGO for 2 and 3 consecutive days was thick (Fig. 2). Furthermore, the RD-10/FD-2000 ratio significantly decreased in rats treated with CG for 1 to 14 consecutive days compared with vehicle-treated rats (Fig. 3a). However, the recovery volume of peritoneal fluid did not decrease in the rats treated with CG for 1 d (Fig. 3b). The peritoneum of rats treated with CG for 2 to 14 d exhibited an altered morphology, but those of rats treated with CG for 1 d and vehicle-treated rats did not change (Fig. 4).

(a) Evaluation of peritoneal injury using the RD-10/FD-2000 ratio, and (b) the residual solution in the peritoneal cavity 120 min after intraperitoneal injection in rats treated with saline or MGO (20 mM) for 1 to 5 consecutive days. The 5-d data are quoted from ref. 17. Each bar represents the mean+S.E. for at least four experiments. *** p<0.001 compared with saline-treated rats.

Observation of the peritoneum of rats treated with saline for (a) 1, (b) 2, and (c) 3 consecutive days. Peritoneum of rats treated with MGO (20 mM) for (d) 1, (e) 2, and (f) 3 consecutive days. Arrows indicate the peritoneum.

(a) Evaluation of peritoneal injury using the RD-10/FD-2000 ratio, and (b) the residual solution in the peritoneal cavity 120 min after intraperitoneal injection in rats treated with 15% ethanol or CG (0.1%) for 1 to 14 consecutive days. Each bar represents the mean+S.E. for at least four experiments. * p<0.05, ** p<0.01, *** p<0.001 compared with 15% ethanol-treated rats.

Observation of the peritoneum of rats treated with 15% ethanol for (a) 1, (b) 2, (c) 3, (d) 5, and (e) 14 consecutive days. Peritoneum of rats treated with CG (0.1%) for (f) 1, (g) 2, (h) 3, (i) 5, and (j) 14 consecutive days. Arrows indicate the peritoneum.
Mice were treated with MGO or CG to develop an easy evaluation method for peritoneal injury. The RD-10/FD-2000 ratio significantly decreased in mice treated with MGO for 1 to 5 consecutive days compared with that in vehicle-treated mice (Fig. 5a). In addition, a high concentration of MGO (25–80 mM) was administered to mice for 1 d because the difference in the RD-10/FD-2000 ratio in mice treated with 20-mM MGO for 1 d was small. The RD-10/FD-2000 ratio significantly decreased in MGO-treated mice and was minimal in those treated with 40-mM MGO (Fig. 5b). Figure 5c shows the RD-10/FD-2000 ratios of the mice treated with CG for 1 to 14 consecutive days. These ratios significantly decreased in the CG-treated mice (Fig. 5c). Moreover, a low concentration of CG (0.01–0.05%) was administered to the mice for 1 d. The RD-10/FD-2000 ratio significantly decreased in mice treated with >0.03% CG (Fig. 5d).

(a) Evaluation of peritoneal injury using the RD-10/FD-2000 ratio in mice treated with MGO (20 mM) for 1 to 5 consecutive days, (b) a high concentration of MGO (25–80 mM) for 1 d, (c) CG (0.1%) for 1 to 14 consecutive days, and (d) a low concentration of CG (0.01–0.05%) for 1 d. Each bar represents the mean+S.E. or ±S.E. for at least four experiments. * p<0.05, ** p<0.01, *** p<0.001 compared with vehicle-treated mice.
To assess whether the RD-10/FD-2000 ratio can be utilized to evaluate drugs that prevent peritoneal injury, thalidomide (which is known to have preventive effects against peritoneal injury) was administered to CG-treated rats. The peritoneum of CG- and thalidomide-treated rats did not exhibit morphologic changes compared with CG-treated rats (Fig. 6). Figure 7 shows the RD-10/FD-2000 ratios in rats treated with CG and thalidomide for 2 to 14 consecutive days. The RD-10/FD-2000 ratio significantly increased in rats treated with CG and thalidomide for 14 consecutive days compared with CG-treated rats.
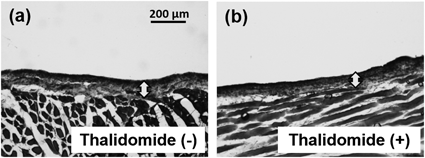
(a) Peritoneum of rats treated with CG (0.1%) for 14 consecutive days. (b) Peritoneum of rats treated with CG (0.1%) and thalidomide for 14 consecutive days. Arrows indicate the peritoneum.

Evaluation of peritoneal injury using the RD-10/FD-2000 ratio in rats treated with CG (0.1%) and thalidomide for 2 to 14 d. Each bar represents the mean+S.E. for at least four experiments. * p<0.05 compared with vehicle-administered rats.
Finally, mice were treated with CG and thalidomide for 1 d to evaluate the drug efficacy of thalidomide using our novel evaluation method. The RD-10/FD-2000 ratio did not increase in mice treated with CG and thalidomide for 1 d compared with CG-treated mice (Fig. 8).

Evaluation of peritoneal injury using the RD-10/FD-2000 ratio in mice treated with CG (0.1%) and thalidomide for 1 d. Each bar represents the mean+S.E. for at least eight experiments. N.S., not significant.
In this study, we evaluated mice and rats for peritoneal injury at an early stage and assessed the efficacy of thalidomide for prevention of peritoneal injury using the RD-10/FD-2000 ratio.
The RD-10/FD-2000 ratio and recovery volume significantly decreased to about 60% in rats treated with MGO for 1 d as well as those treated with MGO for 5 consecutive days (Fig. 1). However, the morphology of the peritoneum in rats treated with MGO for 1 d did not change (Fig. 2d). These results suggest that the RD-10/FD-2000 ratio can be used to detect functional changes in the peritoneum before morphological changes occur. CG is also known to cause peritoneal injury19) and is frequently used to prepare animal models of peritoneal injury. The RD-10/FD-2000 ratio in rats treated with CG for 1 d was also significantly decreased to about 90% (Fig. 3a) before morphological changes occurred (Fig. 4f). However, the recovery volume in rats treaded with CG for 1 d was not decreased (Fig. 3b). These results indicate that the RD-10/FD-2000 ratio can be used to evaluate peritoneal hyperpermeability before the drainage capacity declines and morphological changes occur. In a previous study, transforming growth factor β1 and vascular endothelial growth factor, both of which cause peritoneal fibrosis and angiogenesis, were secreted by rat peritoneal mesothelial cells treated by PDFs for 24 h.20) Thus, morphological changes of the peritoneum may be induced after production of these cytokines, and the RD-10/FD-2000 ratio might therefore be useful for evaluation of peritoneal injury at an early stage.
We prepared a mouse model of peritoneal injury to develop an easier evaluation method than the conventional methods currently in use. Because the body weight of mice is one-tenth that of rats, it is easy to handle mice and decrease the amount of drugs during evaluation of drug efficacy. The RD-10/FD-2000 ratio in mice treated with MGO or CG for 1 d was significantly decreased (Figs. 5a, c). This result suggests that the RD-10/FD-2000 ratio can be used to evaluate peritoneal injury at an early stage in mice. Moreover, when several concentrations of MGO or CG were injected for 1 d, the RD-10/FD-2000 ratio gradually decreased as the concentration of MGO or CG increased (Figs. 5b, d). This suggests that we can induce various degrees of peritoneal injury in mice within a short duration of time.
The use of biocompatible PDFs reportedly has various benefits such as reduction of the epithelial-to-mesenchymal transition in peritoneal mesothelial cells and prevention of peritoneal fibrosis; however, it does not readily prevent peritoneal injury completely.21,22) Thus, it is necessary to develop therapeutic treatments for peritoneal injury. Some studies have suggested that several drugs have therapeutic effects for peritoneal injury.10–16) We evaluated the effect of thalidomide for prevention of peritoneal injury by the RD-10/FD-2000 ratio. Thalidomide is a useful therapeutic drug for peritoneal injury in animal experiments because it prevents peritoneal fibrosis. Moreover, thalidomide has been approved as a therapeutic drug for multiple myeloma and erythema nodosum leprosum, and it is utilized in clinical practice. Although we evaluated peritoneal injury in rats treated by thalidomide following a previous report,15) the thickness of the peritoneum was indistinguishable from that in rats not treated with thalidomide (Fig. 6). However, the RD-10/FD-2000 ratio was significantly increased in rats treated with thalidomide for 14 d (Fig. 7), indicating that the RD-10/FD-2000 ratio can be used to evaluate the drug efficacy of thalidomide, which cannot be distinguished at the morphological level. Here, conventional preparation of animal models of peritoneal injury using MGO (which is a glucose degradation product),23,24) CG,25) or PDF26,27) requires a long duration of time and great effort (MGO is administered for 5 consecutive days, CG is administered for more than 14 consecutive days, and PDF is administered for more than 28 consecutive days). It is thought to be difficult to quickly evaluate drug efficacy for peritoneal injury by conventional evaluation methods. Then, we evaluated the efficacy of short-term administration of thalidomide by the RD-10/FD-2000 ratio. An effect of 1 d of thalidomide administration was not observed in mice (Fig. 8) because CG induced strong peritoneal injury immediately (Fig. 5c). This result suggests that the effects of thalidomide are gradual. Taking all results into consideration, the RD-10/FD-2000 ratio may be used to explore effective drugs for peritoneal injury.
In conclusion, our findings indicate that the RD-10/FD-2000 ratio can be used to evaluate peritoneal injury at an early stage. Moreover, the RD-10/FD-2000 ratio can be used to evaluate the efficacy of thalidomide for prevention of peritoneal injury. Therefore, we might be able to prepare model animals for one day and evaluate drug efficacy for peritoneal injury. This study will contribute to the development of effective treatments for peritoneal injury.
This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan and by the Joint Research Promotion Project of Nagasaki University Graduate School of Biomedical Sciences in 2013.
The authors declare no conflict of interest.