2016 Volume 39 Issue 10 Pages 1646-1652
2016 Volume 39 Issue 10 Pages 1646-1652
Down syndrome (DS), the most common genetic disorder, is caused by trisomy 21. DS is accompanied by heart defects, hearing and vision problems, obesity, leukemia, and other conditions, including Alzheimer’s disease (AD). In comparison, most cancers are rare in people with DS. Overexpression of dual specificity tyrosine-phosphorylation-regulated kinase 1A and a regulator of calcineurin 1 located on chromosome 21 leads to excessive suppression of the calcineurin-nuclear factor of activated T cells (NFAT) signaling pathway, resulting in reduced expression of a critical angiogenic factor. However, it is unclear whether the calcineurin-NFAT signaling pathway is involved in AD pathology in DS patients. Here, we investigated the association between the calcineurin-NFAT signaling pathway and AD using neuronal cells. Short-term pharmacological stimulation decreased gene expression of tau and neprilysin, and long-term inhibition of the signaling pathway decreased that of amyloid precursor protein. Moreover, a calcineurin inhibitor, cyclosporine A, also decreased neprilysin activity, leading to increases in amyloid-β peptide levels. Taken together, our results suggest that a dysregulation in calcineurin-NFAT signaling may contribute to the early onset of AD in people with DS.
Down syndrome (DS) is the most frequent congenital chromosomal disorder, and is primarily caused by an extra copy of chromosome 21. The incidence of DS, approximately one in 600–800 live births, increases with maternal age.1,2) The characteristic physical features of the disease include a flattened face and nose, small head, ears and mouth, wide, short hands with short fingers, dry skin, and decreased or poor muscle tone. Children with DS are at increased risk of complications, including heart defects, hearing and vision problems, obesity, leukemia, and other conditions.3–5) DS patients in middle age are more susceptible to the development of Alzheimer’s disease (AD),6,7) whereas most cancers are rare in people with DS, in whom overall cancer mortality is below 10% of that in the general population.8)
Vascular endothelial growth factor (VEGF) plays a central role in tumor development.9,10) VEGF and the calcineurin-nuclear factor of activated T cells (NFAT) signaling pathway regulates cancer metastasis.9) The Ca2+/calmodulin-dependent serine/threonine phosphatase calcineurin dephosphorylates NFAT in the cytoplasm, leading to its nuclear translocation and activation.9,11,12) Dual specificity tyrosine-phosphorylation-regulated kinase 1A (DYRK1A) phosphorylates NFAT in the nucleus, leading to its cytoplasmic translocation and inactivation.9,12) Calcineurin is negatively regulated by the DS critical region protein regulator of calcineurin 1 (RCAN1) in the cytoplasm.9,12) The genes for both DYRK1A and RCAN1 are located on chromosome 21. The calcineurin-NFAT signaling pathway is perturbed by the increased dosage of DYRK1A and RCAN1 in DS patients,13) and this disruption reduces the incidence of solid tumors in these patients.
Calcineurin-NFAT signaling plays a role not only in tumor progression, but also in the immune response, bone homeostasis, the development of cardiac and skeletal muscle, and in the nervous system.12,14) The etiology of DS results in part from dysfunction of calcineurin-NFAT signaling. However, it is unclear whether the calcineurin-NFAT signaling pathway is involved in AD pathology in DS patients.
In this study, we investigated the role of the calcineurin-NFAT signaling pathway in AD pathology. We pharmacologically modulated this pathway in neuronal cells and examined changes in the expression of AD-related genes, including those encoding tau (microtubule-associated protein tau, MAPT), a major component of neurofibrillary tangles, amyloid precursor protein (APP), a precursor of amyloid-β peptide (Aβ) which is a major component of senile plaques, and neprilysin (NEP; also known as membrane metallo-endopeptidase (MME), CD10, and CALLA), a major Aβ-degrading enzyme in the brain.15) Although there was no change in APP expression, there were significant reductions in expression of tau and NEP in human neuronal SH-SY5Y cells treated with calcineurin-NFAT signaling activators. In contrast, the treatment with a calcineurin inhibitor, cyclosporine A, in SH-SY5Y cells caused a decrease in APP expression without affecting gene expression of tau and NEP.
Furthermore, we found that the calcineurin-NFAT signaling pathway affected both NEP activity and Aβ levels. These results suggest that a malfunction in the calcineurin-NFAT signaling pathway may contribute to the early onset of AD in people with DS.
Cyclosporin A, FK506, and 12-O-tetradecanoylphorbol-13-acetate (TPA) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.), Enzo Life Sciences (Farmingdale, NY, U.S.A.), and Cell Signaling Technology (Danvers, MA, U.S.A.), respectively, and were dissolved in dimethyl sulfoxide. Ionomycin was purchased from Cayman Chemical Company (Ann Arbor, MI, U.S.A.), and diluted with ethanol.
PlasmidThe pGL4.30[luc2P/NFAT-RE/Hygro] vector, containing an NFAT response element located upstream of the luciferase reporter gene luc2P, was purchased from Promega (Madison, WI, U.S.A.).
Cell CultureHuman neuroblastoma SH-SY5Y cells were obtained from European Collection of Authenticated Cell Cultures (ECAC C; Salisbury, U.K.) and maintained in Dulbecco’s modified Eagle’s medium (Nacalai Tesque, Kyoto, Japan) supplemented with 10% heat-inactivated fetal bovine serum (SAFC Biosciences, Lenexa, KS, U.S.A.), 100 U/mL penicillin and 100 µg/mL streptomycin (Nacalai Tesque) at 37°C in a humidified 5% CO2 incubator.
Stable SH-SY5Y cell lines expressing a reporter vector (pGL4.30[luc2P/NFAT-RE/Hygro]) or a human APP695 vector (pcDNA3.1 containing APP695) were generated by transient transfection followed by antibiotic selection with hygromycin B (Wako Pure Chemical Industries, Ltd., Osaka, Japan) or G418 (Nacalai Tesque) and cloning.
Luciferase Reporter AssayA stable SH-SY5Y cell line containing a reporter construct (pGL4.30[luc2P/NFAT-RE/Hygro]) was grown for 24–66 h, and then treated with 1 µM ionomycin and 10 ng/mL TPA to stimulate the calcineurin-NFAT signaling pathway for the indicated time. Cells treated with the compounds were harvested and lysed in Glo Lysis Buffer (Promega). The activity of luc2P, a synthetically-derived luciferase with humanized codon optimization, as indicated by relative luminescence units, was determined using the ONE-Glo Luciferase Assay System (Promega) according to the manufacturer’s instructions, and assessed in a 384-well plate reader (Cytation 3; BioTek, Winooski, VT, U.S.A.). Luminescent intensities were normalized by protein amounts of cell lysates. Protein concentration was determined using a bicinchoninic acid (BCA) protein assay kit (Takara Bio, Shiga, Japan).
Quantitative Real-Time PCRTotal RNA was purified from SH-SY5Y cells treated with the calcineurin-NFAT pathway stimulants for 6 h using the High Pure RNA Isolation Kit (Roche Diagnostics, Mannheim, Germany). For quantitative real-time PCR, 200 ng of total RNA was reverse transcribed using PrimeScript RT Master Mix (Takara Bio) and assessed by quantitative real-time PCR using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA as an internal control. All primers (Table 1) were purchased from Takara Bio. PCR amplifications were performed using SYBR Premix Ex Taq (Tli RNaseH Plus) (Takara Bio). Thermocycling (95°C for 5 s, followed by 40 cycles of 95°C for 5 s and 60°C for 20 s) was performed on a Smart Cycler II System (Cepheid, Sunnyvale, CA, U.S.A.).
| Gene | Protein | Reference sequence | Primer sequence | Product size (bp) |
|---|---|---|---|---|
| APP | APP | NM_000484.3 | F: 5′-GGGTTCAAACAAAGGTGCAATC-3′ | 177 |
| R: 5′-TGCTGCATCTTGGACAGGTG-3′ | ||||
| Tau (MAPT) | Tau | NM_016835.4 | F: 5′-TCTCCAAGTAAAGCCACGAGGTC-3′ | 133 |
| R: 5′-TAGGCCAGTGCCCAGGGTAA-3′ | ||||
| NEP (MME) | Neprilysin | NM_000902.3 | F: 5′-GGGAGCTGATGAAACTCACAAATG-3′ | 142 |
| R: 5′-TCTCTGGACAGCTTGCACCTAC-3′ | ||||
| RCAN1 | RCAN1 | NM_004414.6 | F: 5′-AGCACTTGCTTGCGGAACTC-3′ | 68 |
| R: 5′-AGTTACACGTTGCACGGTTGG-3′ | ||||
| GAPDH | GAPDH | NM_002046.5 | F: 5′-GCACCGTCAAGGCTGAGAAC-3′ | 138 |
| R: 5′-TGGTGAAGACGCCAGTGGA-3′ |
F and R mean forward (sense) and reverse (antisense) primers, respectively.
Cellular neprilysin activity was measured as previously described with modification.16) Briefly, SH-SY5Y cells stably transfected with APP695 were treated with the calcineurin-NFAT pathway activators for 6 h or with cyclosporine A for 72 h, and then harvested and lysed in a buffer containing 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (pH 7.4), 150 mM NaCl, 0.5% Triton X-100, 1 µM pepstatin A (Sigma-Aldrich, St. Louis, MO, U.S.A.) and protease inhibitor cocktail, ethylenediaminetetraacetic acid (EDTA)-free (cOmplete; Roche Diagnostics) on ice. The cell lysate was freeze-thawed at three 20-min intervals and centrifuged at 14000×g for 20 min at 4°C. The supernatant protein concentrations were determined using a BCA protein assay kit. NEP-dependent neutral endopeptidase activity in the cell lysate was fluorometrically assayed using an indirect coupled enzyme assay method. The standard assay mixture consisted of 5 µg of cell lysate, 0.1 mM succinyl-Ala-Ala-Phe-MCA (Bachem AG, Bubendorf, Switzerland) as a substrate, and 100 mM 2-(N-morpholino)ethanesulfonic acid (MES) (pH 6.5) in a total volume of 50 µL. The reaction was initiated by addition of substrate to the assay mixture, and performed for 1 h at 37°C. Then, leucine aminopeptidase (Sigma-Aldrich) and 0.2 mM phosphoramidon (Peptide Institute, Osaka, Japan) were added and incubated for 30 min at 37°C to remove the phenylalanine residue from the Phe-MCA generated by the neutral endopeptidase. The intensity of the liberated 7-amino-4-methylcoumarin was measured with excitation at 390 nm and emission at 460 nm on a 96-well half-area-black plate using a microplate spectrometer (Infinite M-1000; Tecan, Männedorf, Switzerland). The NEP-dependent neutral endopeptidase activity was determined based on the decrease in the rate of digestion produced by 10 µM thiorphan (Sigma-Aldrich), a specific NEP inhibitor.
Sandwich Enzyme-Linked Immunosorbent Assay (ELISA) for AβThe concentration of Aβ40 and Aβ42 in the culture medium of SH-SY5Y cells stably transfected with APP695 and treated with 1 µM ionomycin and 10 ng/mL TPA for 6 h or cyclosporine A for 72 h were determined by sandwich ELISA using the monoclonal antibody BAN50 as a capture antibody for total Aβ and BA27 or BC05 as detection antibodies for Aβ40 or Aβ42, respectively.17)
Statistical AnalysisAll values were expressed as the mean±standard deviation (S.D.). For comparisons between two groups, a one-tailed or two-tailed Student’s t-test was used. For comparisons among more than three groups, a Student–Newman–Keuls multiple comparison test was used. A difference was considered significant if the p-value was less than 0.05.
The calcineurin-NFAT signaling pathway is up-regulated by increases in intracellular Ca2+ levels. A Ca2+ ionophore, ionomycin, and a phorbol ester, TPA, are commonly used to stimulate calcineurin-NFAT signaling.18) We generated an SH-SY5Y cell line stably transfected with the luciferase reporter gene luc2P driven by an NFAT response element. Using this cell line, we conducted time-course analysis of the calcineurin-NFAT promoter activity by measuring luciferase activity (Fig. 1a). In agreement with previous reports,19) we found that treatment with ionomycin and TPA strongly up-regulated luciferase activity in our cell culture system. When we measured luciferase activities between 3 and 48 h after the addition of ionomycin and TPA, the maximum luciferase activity was observed at 6 h, therefore we decided to examine at 6 h (short-term) for subsequent analyses of the calcineurin-NFAT signaling pathway.
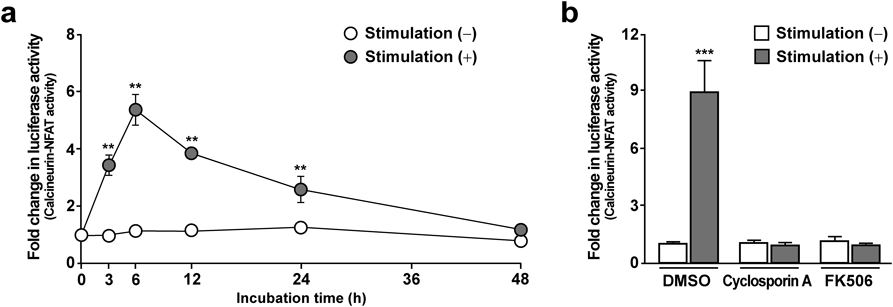
SH-SY5Y cells stably transfected NFAT response element and a luciferase reporter gene luc2P were treated with 1 µM ionomycin and 10 ng/mL TPA for the indicated time (a). SH-SY5Y cells stably transfected NFAT response element and a luciferase reporter gene luc2P were treated with 1 µM ionomycin and 10 ng/mL TPA in addition of 1 µM cyclosporin A or 1 µM FK506 for 6 h (b). The calcineurin-NFAT promoter activities were determined by luciferase reporter assay. Data represent the mean±S.D. (n=4, representative data is shown). All values are normalized to protein amounts of cell lysates. ** p<0.01 and *** p<0.001: significantly different from the unstimulated group.
To confirm that the increase in luciferase activity produced by treatment with ionomycin and TPA was mediated by the calcineurin-NFAT signaling pathway, we added calcineurin inhibitors (cyclosporine A, FK506), which are used clinically as an immunosuppressive drug along with the calcineurin-NFAT pathway activators (Fig. 1b). Both cyclosporine A and FK506 completely abolished the increase in luciferase activity elicited by the calcineurin-NFAT activators. This finding indicates that the calcineurin-NFAT signaling pathway is intact and functional in human neuronal SH-SY5Y cells.
RCAN1 Expression Is Up-Regulated by Calcineurin-NFAT Pathway ActivatorsRCAN1 expression is regulated by the calcineurin-NFAT signaling pathway in cardiac,20) skeletal,21) and endothelial22) cells. Up-regulation of RCAN1 may function to feedback inhibit the calcineurin-NFAT signaling pathway to suppress calcineurin.23) Therefore, we assessed RCAN1 expression in SH-SY5Y cells following treatment with the calcineurin-NFAT signaling activators by real-time PCR (Fig. 2). RCAN1 expression was significantly up-regulated 6 h after treatment with ionomycin and TPA, compared with untreated cells.
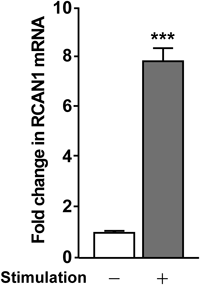
SH-SY5Y cells were treated with 1 µM ionomycin and 10 ng/mL TPA for 6 h. mRNA levels of RCAN1 were determined by real-time PCR analysis. All values are normalized to GAPDH. Data represent the mean±S.D. (n=3, representative data is shown). *** p<0.001: significantly different from the unstimulated group.
To investigate the relationship between the AD-related genes APP, tau, and NEP and the calcineurin-NFAT signaling pathway, we measured their mRNA levels in SH-SY5Y cells treated with the calcineurin-NFAT signaling activators in 6 h (short-term) (Figs. 3a–c). There was no significant difference between unstimulated control cells and stimulated cells in expression levels of APP (97.6±10.5%, p=0.84). In contrast, decreases in expression levels of tau (64.3±6.8%, p<0.05) and NEP (61.5±11.8%, p<0.05) were observed after treatment with the calcineurin-NFAT signaling activators.

SH-SY5Y cells were treated with 1 µM ionomycin and 10 ng/mL TPA for 6 h or 1 µM cyclosporine A. mRNA levels of APP (a, d), tau (b, e), and NEP (c, f) were determined by real-time PCR analysis. All values are normalized to GAPDH. Data represent the mean±S.D. (n=3–5, representative data is shown). * p<0.05: significantly different from the unstimulated group.
To investigate the long-term inhibitory effect of calcineurin-NFAT signaling pathway on expression of APP, tau, and NEP, we measured their mRNA levels in SH-SY5Y cells treated with cyclosporine A for 72 h (Figs. 3d–f). There was no significant difference between unstimulated control cells and stimulated cells in expression levels of tau (107.8±12.9%, p=0.30) and NEP (95.9±7.0%, p=0.31). In contrast, a decrease in APP expression level (90.2±5.0%, p<0.05) was observed. Although treatment with the calcineurin-NFAT signaling activators caused no change of APP expression, treatment with the calcineurin-NFAT signaling inhibitor caused reduced APP expression.
Neprilysin Activity Tends to Be Reduced by the Calcineurin-NFAT Signaling ActivatorsWe next investigated NEP activity in cells treated with the calcineurin-NFAT signaling activators ionomycin and TPA. As shown in Fig. 4a, the calcineurin-NFAT activators tended to reduce NEP activity by 15% in SH-SY5Y cells. In comparison, these activators caused a significant (45%) decrease in NEP activity in non-neuronal HEK293 cells (data not shown).
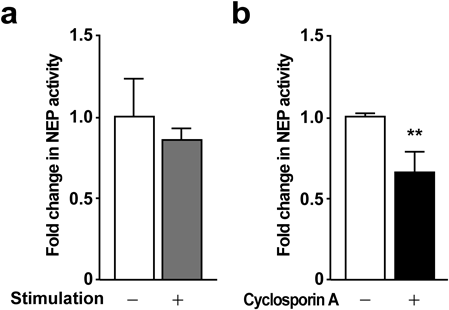
SH-SY5Y cells stably transfected APP695 were treated with 1 µM ionomycin and 10 ng/mL TPA for 6 h (a). SH-SY5Y cells stably transfected APP695 were treated with 1 µM cyclosporin A for 72 h (b). Neprilysin activities were determined by an indirect coupled enzyme assay with a specific fluorescent substrate. Data represent the mean±S.D. (n=4, representative data is shown). ** p<0.01: significantly different from the vehicle-treated group.
The calcineurin-NFAT signaling pathway is suppressed in people with DS because of overexpression of RCNA1 and DYRK1A. To examine the effect of long-term suppression of calcineurin-NFAT signaling on NEP activity, we treated SH-SY5Y cells with cyclosporin A for 72 h (Fig. 4b). This long-term treatment with cyclosporine A caused a substantial reduction in NEP activity (65.8±12.1%, p<0.01).
Aβ40 and Aβ42 Levels Are Increased by Short-Term Stimulation or Long-Term Suppression of the Calcineurin-NFAT Signaling PathwayTreatment with the calcineurin-NFAT signaling activators for 6 h (short-term) or with the calcineurin inhibitor for 72 h (long-term) caused the down-regulation of NEP activity. NEP is a major Aβ-degrading enzyme, and Nep-deficient mice show increased Aβ in the brain.15) Because Aβ plays a critical role in AD pathogenesis,24) we subsequently measured Aβ40 and Aβ42 levels in the conditioned medium of SH-SY5Y cells stably overexpressing APP treated with ionomycin and TPA (Fig. 5a) or cyclosporine A (Figs. 5b, c). Following short-term treatment, although increased Aβ40 level was observed, Aβ42 level fell below the detection limit. In contrast, long-term treatment with the calcineurin-NFAT signaling pathway inhibitor increased both Aβ40 and Aβ42 levels by approximately 40 and 90%, respectively.

SH-SY5Y cells stably transfected APP695 were treated with 1 µM ionomycin and 10 ng/mL TPA for 6 h (a). SH-SY5Y cells stably transfected APP695 were treated with 1 µM cyclosporin A for 72 h (b, c). The amounts of Aβ40 and Aβ42 in conditioned medium were determined by sandwich ELISA. Data represent the mean±S.D. (n=5, representative data is shown). * p<0.05 and *** p<0.001: significantly different from the vehicle-treated group.
The NFAT family of transcription factors, first identified as regulators of T-cell activation, consists of five members—NFAT1 (NFATc2 or NFATp), NFAT2 (NFATc1 or NFATc), NFAT3 (NFATc4), NFAT4 (NFATc3 or NFATx), and NFAT5 (TonEBP or OREBP).9,11,25) With the exception of NFAT5, these proteins are regulated by intracellular Ca2+ and by phosphorylation/dephosphorylation.9,11,25) In the steady state, phosphorylated NFATs are localized to the cytoplasm in an inactive form, and calcineurin, activated by Ca2+ flux, directly dephosphorylated NFATs, leading to its transfer into the nucleus in an active form. In hippocampal and cerebellar regions of mild cognitive impairment (MCI) and AD brains, the levels of NFATs were changed in a region- and pathology-specific manner.26) Nuclear NFAT1 level was increased in MCI brains, but decreased slightly in AD brains, in contrast nuclear NFAT3 was increased in AD and decreased slightly in MCI without any changes in NFAT2 level. These findings suggest that possible isoforms of NFAT may contribute to AD development.26) Calcineurin, a Ca2+ activated protein serine/threonine phosphatase, is abundant in the central nervous system.27,28) In addition to NFATs, calcineurin substrates include Bcl-2 associated death protein (BAD),29) N-methyl-D-aspartate (NMDA) receptor,30) glycogen synthase kinase-3β (GSK-3β),31) and tau32) in neuronal cells. Calcineurin activity is also regulated by an endogenous inhibitor, RCAN1. Calcineurin hyperactivity causes memory impairment, neuroinflammation and neuronal death, all of which are pathological features of AD.27)
Recent studies using neuronal cells demonstrate that NFAT1 modulates the expression of the beta-site APP cleaving enzyme 1 (BACE1) gene, encoding an aspartic protease involved in Aβ production,33) and that NFAT3 binding on the BACE1 promoter region enhances BACE1 expression.34) Nevertheless, the association between the calcineurin-NFAT signaling pathway and AD is poorly understood. In the present study, we demonstrated that calcineurin-NFAT signaling activators decreased expression of tau and NEP without affecting APP gene expression (Figs. 3a–c). NAFT may act as a gene repressor of tau and NEP gene expression, because NFAT1 could directly bind with the cyclin A2 promoter.35) In contrast, the treatment with a calcineurin inhibitor, cyclosporine A, caused a decrease in APP gene expression without any changes in tau and NEP genes expression (Figs. 3d–f). These results suggest that the genes expression is intricately regulated by several gene transcriptional factors. Indeed, it has been reported that tau gene expression was regulated by protein kinase A-cAMP response binding protein (CREB) signaling pathway36) and that calcineurin dephosphorylated phosphorylated CREB, to inhibit its transcriptional activity.37) Because the calcineurin-NFAT signaling pathway is suppressed by the overexpression of RCAN1 and DYRK1A in DS, tau expression might be increased in these patients. Interestingly, tau mRNA as well as APP mRNA levels are increased in the brains of DS patients.38) Moreover, calcineurin phosphatase activity associates tau protein metabolism as well as mRNA transcription, and inhibition of calcineurin activity by cyclosporine A or FK506 increases extracellular total tau and intracellular phosphorylated tau.39) Tau is a principal component of neurofibrillary tangles, and is required for neurotoxicity induced by Aβ.40) Furthermore, murine wild-type tau transgenic mice show a progressive increase in hyperphosphorylated tau pathology with age.41) Increased tau expression likely accelerates AD pathology in people with DS. The treatment with calcineurin-NFAT signaling activators caused a decrease in tau gene expression (Fig. 3b). It is proposed that modulation of total tau level would be one of therapeutic targets for AD.42) Because human tau gene undergoes alternative splicing to produce multiple isoforms with three (3R) or four (4R) repeats in the microtubule binding domain and the ratio between 4R and 3R tau may be a key for tau-related neurodegeneration including AD,43) it is required to investigate the effect of calcineurin-NFAT signaling activator on alternative splicing of tau heterogeneous nuclear RNA to assess as an approach to treat AD.
RCAN1, an endogenous regulator of the calcineurin-NFAT signaling pathway, blocks calcineurin-dependent dephosphorylation of NFAT in endothelial cells, and its overexpression potently inhibits tumor angiogenesis in DS patients.9) Expression of RCAN1 is regulated by the calcineurin-NFAT signaling pathway in a negative feedback loop in various cells.20–22) Similarly, RCAN1 functions as an inhibitor of the calcineurin-NFAT signaling pathway in neuronal cells (Fig. 2). RCAN1 is a mediator of stress and Aβ-induced neuronal death, and overexpression of RCAN1 activates caspase-3 and induces neuronal apoptosis.44) Furthermore, oxidative stress induces RCAN1 expression in primary neurons.45) Aβ causes neuronal cell death by enhancing oxidative stress and disrupting cellular calcium homeostasis in the AD brain.46) Transient overexpression of RCAN1 induced by perturbation of the calcineurin-NFAT negative feedback loop might protect cells against oxidative stress and Ca2+ overload, leading to chronic RCAN1 overexpression, thereby exacerbating AD pathology in a calcineurin-dependent or -independent manner.47) As a consequence, DS patients would be at risk of neurodegeneration through neuronal apoptosis from persistent overexpression of RCAN1.
In this study, the calcineurin-NFAT signaling pathway seems to have some influence on the major pathological hallmark of AD, i.e., Aβ and tau (Figs. 3–5). In addition to decreased gene expression of tau, the short-term treatment with the calcineurin-NFAT signaling activators decreased expression of NEP gene (Fig. 3c). Interestingly, whereas NEP activity tended to be reduced by short-term treatment with the calcineurin-NFAT signaling activators (Fig. 4a), long-term pharmacological inhibition of calcineurin with cyclosporine A caused a reduction in NEP activity (Fig. 4b). NEP protein in the brains of wild-type or APP transgenic mouse is reported to be metabolically stable.48) This facts may explain that there was a poor correlation between NEP mRNA level and NEP activity in neuronal SH-SY5Y cells in the present study. NEP is a major Aβ-degrading enzyme, and numerous in vivo and in vitro studies show a link between NEP and AD pathology.15,16,49) NEP mRNA, protein and activity levels decrease with age in the cortex and hippocampus of rodents and humans, and are also reduced in the AD brain.50) Taken together with these previous observations, our findings (Fig. 3c) suggest that the calcineurin-NFAT signaling pathway, activated by Ca2+ influx, reduces NEP expression in neurons in AD patients, particularly as these cells have impaired Ca2+ homeostasis. Equally important, as seen in individuals with DS, prolonged inhibition of calcineurin-NFAT signaling also reduces NEP activity (Fig. 4), resulting in increases in Aβ40 and Aβ42 (Figs. 5b, c). Accordingly, perturbed calcineurin-NFAT signaling in DS patients may be a strong risk factor for the development of amyloid pathology. Furthermore, recipients of immunosuppressive drugs, such as implanted patients, may also have a potential risk of AD similar to DS patients.
In conclusion, our present results provide in vitro evidence that a disruption in the calcineurin-NFAT signaling pathway reduces expression of tau and NEP, resulting in increased Aβ levels. NEP degrades Aβ very efficiently, and a decrease in its expression or activity would shift the balance between Aβ production and degradation. Further studies are required to clarify the molecular mechanisms underlying the regulation of tau and NEP gene expression by NFAT and related factors. Nonetheless, our findings suggest that dysfunction of the calcineurin-NFAT signaling pathway might contribute to the early onset of AD in people with DS.
The authors thank Prof. Masaaki Kai (Nagasaki University) for his valuable advice as a mentor. This work was supported by the Promotion and Standardization of the Tenure-Track System from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
The authors declare no conflict of interest.