2016 年 39 巻 10 号 p. 1687-1693
2016 年 39 巻 10 号 p. 1687-1693
A novel sustained release formulation of mitomycin C (MMC) was developed by employing single-walled carbon nanotubes (SWCNTs) wrapped by designed peptide with polyethylene glycol (PEG) modification (pegylation) as a nano-scale molecular platform. The amino groups of polycationic and amphiphilic H-(-Cys-Trp-Lys-Gly-)(-Lys-Trp-Lys-Gly-)6-OH [CWKG(KWKG)6] peptide associated with SWCNTs were modified using PEG with 12 units (PEG12) to improve the dispersion stability of the composite. Then thiol groups of peptide were conjugated with MMC using N-ε-maleimidocaproic acid (EMCA) as a linker via transformation of aziridine group of MMC. The obtained SWCNTs-CWKG(KWKG)6-(PEG)12-C6-MMC composites particularly that with 13.6% PEG modification extent of amino groups, showed good dispersion stability both in water and in a cell culture medium for 24 h. The release of MMC from SWCNTs-CWKG(KWKG)6-(PEG)12-C6-MMC was confirmed to follow first-order kinetics being accelerated by the pH increase in good agreement with the results observed for MMC-dextran conjugate with the same conjugation structure. The SWCNTs-CWKG(KWKG)6-(PEG)12 composite exhibited a considerably low cytotoxicity against cultured human lung adenocarcinoma epithelial cell line (A549). In contrast, SWCNTs-CWKG(KWKG)6-(PEG)12-C6-MMC demonstrated delayed but relatively corresponding antitumor activity with free MMC at the same concentration. The results suggested the potential role of SWCNTs-CWKG(KWKG)6-(PEG)12 as a carrier for a controlled release drug delivery system (DDS).
Optimized drug delivery and the resulting improved drug efficiency imply an efficient and selective delivery of a drug to its site of action and reduced drug exposure on the non-target sites.1) This is a basic rationale for concept of controlled drug delivery, i.e., the use of systems and techniques for altering and controlling the disposition and cellular uptake of pharmacologically active agents. It is particularly important in cancer chemotherapy to find ways to control the pharmacokinetic drug behavior as most anticancer agents show unselective cytotoxicity and severe side effects. Therefore, considerable research was conducted to develop delivery systems for anticancer agents.
The use of a physical device or the chemical transformation of a drug molecule into an inactive latent form is a promising approach for improving the delivery of anticancer agents. Previous studies examined drug delivery system (DDS) for cancer from viewpoints of utilizing physical devices such as emulsion,2) gelatin microspheres,3) liposomes,4) bubble liposomes5,6) as well as chemical modification of drug molecules.7) For example, prior research developed the macromolecular derivative of mitomycin C (MMC) utilizing water-soluble dextran derivatives with various linkers between aziridine group of MMC and hydroxyl group of dextran.8) The obtained MMC-linker-dextran derivatives offered suitable in vivo bio-distribution patterns due to their physicochemical and biological properties with the controlled release of MMC.9,10)
In contrast, a novel composite of single-walled carbon nanotubes (SWCNTs) with amphiphilic designed peptides was developed and their chemical and physicochemical characteristics were evaluated with respect to biomedical applications.11) SWCNTs are one of the most attractive materials in the biomedical field due to their unique cylindrical structure with broad surface areas and physical, chemical, electrical, and mechanical properties.12,13) In addition, SWCNTs are prominently taken up into cells in vitro via direct penetration or endocytic pathway.14,15) They may also accumulate in the tumor tissue via enhanced permeability and retention (EPR) effect16) after intravenous administration. In SWCNTs composites wrapped with amphiphilic H-(-Lys-Trp-Lys-Gly-)7-OH [(KWKG)7)] peptides, the (KWKG)7 peptide interacts with the surface of SWCNTs via π–π interaction at multiple points between the indole skeleton of the tryptophan residue and SWCNTs.11) Hence, SWCNTs-(KWKG)7 shows good dispersion stability in water because of electrostatic repulsion between themselves. Moreover, polyethylene glycol (PEG) modifications of the SWCNTs-(KWKG)7 composites remarkably improved the dispersion stability even under a biological environment, thereby suggesting the tremendous potential of these composites as drug and gene carriers.17)
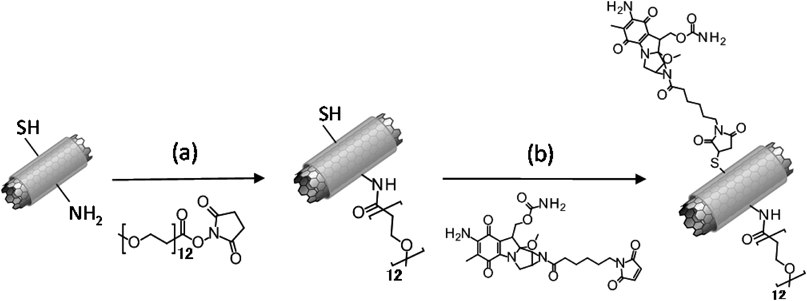
In this study, SWCNTs composites with CWKG(KWKG)6 peptides having thiol and amino groups were employed and MMC was conjugated to the thiol groups employing N-ε-maleimidocaproic acid (EMCA) as a linker to transform the aziridine group of MMC after pegylation of amino groups. The controlled release characteristics and cytotoxic activities of the SWCNTs-CWKG(KWKG)6-PEG12-C6-MMC composites are discussed.
Purified SWCNTs [HiPco18); Lot No. P0343] were obtained from Carbon Nanotechnologies (Houston, TX, U.S.A.). The CWKG(KWKG)6 peptide shown in Fig. 1 was synthesized with more than 90% purity by Invitrogen (Carlsbad, CA, U.S.A.). The SWCNTs-CWKG(KWKG)6 composite was prepared in 5 mL D2O solution containing 2 mg SWCNTs and 10 mg CWKG(KWKG)6 peptide in a glass tube by sonicating for 1 h with an ultrasonic disruptor UD-201 (TOMY Digital Biology, Tokyo, Japan) in an ice water bath.11,17) The chemical and structural stability of peptides during sonication was demonstrated in our previous study, where a complex formation of peptides with SWCNTs was indicated by atomic force microscopy (AFM) image of SWCNTs-peptide and β-sheet formation of peptide was assigned by circular dichroism (CD) spectrum.11) D2O solution was employed to prepare density difference between dispersed and non-dispersed SWCNTs D2O. After the sonication, the dispersion was centrifuged at 40000 rpm for 2.5 h with a Himac CP65β ultracentrifuge and a model P40ST rotor (Hitachi, Tokyo, Japan). The supernatant of SWCNTs-CWKG(KWKG)6 dispersion was dialyzed using a 100 kD dialysis membrane tube (Spectrum Laboratories Inc., CA, U.S.A.) against distilled water for 24 h to remove free unbounded CWKG(KWKG)6 peptide.

The SWCNTs concentration of the SWCNTs-CWKG(KWKG)6 dispersion was determined by the absorbance spectrum obtained at 808 nm with A1 mg/mL=40.312) using a UV spectrophotometer (UV-1600, Shimadzu, Kyoto, Japan). The CWKG(KWKG)6 peptide concentration was quantified at 280 nm with A280 nm=5500 M−1 cm−1 as an extinction coefficient of tryptophan residue.19)
PEG Modification of SWCNTs-CWKG(KWKG)6The PEG modification of the amino groups of lysine residue of SWCNTs-CWKG(KWKG)6 was performed by covalent amide bond formation between amino groups and carboxyl groups of succinimidyl-[(N-methyl)-dodecaethyleneglycol]ester [methyl-(PEG)12-NHS ester, MS(PEG)12; Thermo Fisher Scientific, Rockford, IL, U.S.A.] pegylation reagent.20) The SWCNTs-CWKG(KWKG)6 dispersion in pH 8.0 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer (25 mM) was added along with various volumes of 2.5 mM MS(PEG)12 to set the molar ratio at 10, 20, 30, 40 and 50% against the total amino groups. The reaction was performed for 2 h, and the reaction solution was filtered by centrifugation three times using an Amicon Ultra-0.5 Centrifugal Filter 100 kD Device (Merck Millipore Ltd., Germany) for exchanging the buffer and removing free unconjugated MS(PEG)12. The extent of PEG modification of amino groups was determined as reported in a previous study17) using fluorescein isothiocyanate (FITC)-(PEG)12-NHS ester and was calculated from the absorbance spectrum and molar extinction coefficient of FITC (494 nm and A494 nm=68000 M−1 cm−1) in the FITC-(PEG)12-modified SWCNTs-CWKG(KWKG)6. In this study, the extents of PEG modification are expressed based on the results of this experiment under the same reaction condition.
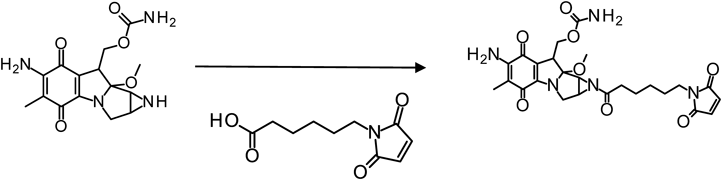
Synthesis of MMC-EMCAAs the first step of conjugation, the aziridine group of MMC (Wako Pure Chemical Industries, Ltd., Osaka, Japan) was coupled to EMCA (Thermo Fisher Scientific) containing both maleimide group and carboxyl group via a carbodiimide reaction (Fig. 2). EMCA (40 mM) and 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride (EDC) (120 mM) were mixed in N,N-dimethylformamide (DMF) for 30 min. Then pH 5.5 2-(N-morpholino)ethanesulfonic acid (MES) buffer (25 mM) was added. Furthermore, MMC was immediately added to the reaction solution at a concentration of one-third of EMCA and reacted for 3 h. The reacted product was purified with reverse phase HPLC with the gradient mode from 10 to 70% of acetonitrile concentration percentage for 20 min. The objective fraction solution was freeze-dried and dissolved in DMF.
The MMC-EMCA concentration measurement was carried out using 4,4ʹ-dithiodipyridine (4-PDS). MMC-EMCA was reacted with an excessive amount of 2-aminoethanethiol (cysteamine) (Fig. 3A). Then, the unreacted residual cysteamine was reacted with 4-PDS (Fig. 3B). The absorbance at 324 nm with A324 nm=19800 M−1 cm−1 21) was compared with that of the control reaction solution without MMC-EMCA.

The concentration of MMC-EMCA was determined based on the differences between the optical absorbance of 4-mercaptopyridine at 324 nm following the reaction of (A) and (B).
In order to prepare SWCNTs-CWKG(KWKG)6-(PEG)12-C6-MMC (Fig. 4), the PEG modification of the amino groups was performed and this was followed by MMC-EMCA conjugation to the thiol groups of SWCNTs-CWKG(KWKG)6. MMC-EMCA with a concentration equal to five times that of the SWCNTs composite thiol groups was added to the SWCNTs-CWKG(KWKG)6-(PEG)12 purified by centrifugal filtration and dispersed in a pH 6.5 MES buffer, and then reacted for 5 h. After the reaction, the obtained SWCNTs-CWKG(KWKG)6-(PEG)12-C6-MMC was filtered by centrifugation three times for a buffer exchange to pH 5.5 25 mM MES buffer. The absorbance spectrum of SWCNTs-CWKG(KWKG)6-(PEG)12-C6-MMC was obtained and the MMC modification extent was determined with absorbance at 364 nm and A364 nm=22000 M−1 cm−1 of the molar extinction coefficient of MMC.22)
The dispersion stability of the SWCNTs-CWKG(KWKG)6 complex with or without pegylation and/or MMC conjugation in water and the cell culture medium was evaluated by visual observation. The SWCNTs-CWKG(KWKG)6 dispersion with various modification conditions was incubated for 24 h under 5% CO2 at 37°C and visually observed for 24 h.17)
Determination of Release Rate of MMC from SWCNTs-CWKG(KWKG)6-(PEG)12-C6-MMCThe SWCNTs-CWKG(KWKG)6-(PEG)12-C6-MMC dispersion in a pH 5.5 MES buffer (25 mM) was exchanged by centrifugal filtration that was performed three times with isotonic pH 5.0 acetate buffer, pH 6.0 MES buffer, pH 7.0, or pH 8.0 HEPES buffer. After the preparation of each dispersion solution, the SWCNTs-CWKG(KWKG)6-(PEG)12-C6-MMC dispersion was incubated at 37°C, and the supernatant obtained at different time periods by centrifugal filtration with a Durapore® (PVDF) 0.22 µm membrane filter (Merck Millipore Ltd.) was tested. The amount of released MMC was determined by obtaining the absorbance of the filtrated solution at 364 nm. The confirmation of the released compound from SWCNTs-CWKG(KWKG)6-(PEG)12-C6-MMC was carried out by reverse phase HPLC with the gradient mode from 10 to 70% acetonitrile concentration for 20 min. The objective fraction was compared with that of the free MMC.
Cell Culture and in Vitro Assay for Cytotoxicity and Antitumor ActivityThe human lung adenocarcinoma epithelial cell line (A549) was obtained from the European Collection of Cell Cultures (ECAC C, Salisbury, U.K.) and cultured in Dulbecco’s modified Eagle’s medium (DMEM; Nissui Pharmaceutical, Tokyo, Japan) under 5% CO2 at 37°C. The culture medium was supplemented with 10% fetal bovine serum (FBS; MP Biochemicals, Irvine, CA, U.S.A.), 100 IU/mL penicillin and 100 µg/mL streptomycin.
The cytotoxicity of SWCNTs-CWKG(KWKG)6-(PEG)12 and the antitumor activity of SWCNTs-CWKG(KWKG)6-(PEG)12-C6-MMC were evaluated by WST-1 assay reagent (TaKaRa Bio, Shiga, Japan). In these experiments, the PEG modification extent of the amino groups was fixed at 13.6% and the MMC concentration of SWCNTs-CWKG(KWKG)6-(PEG)12-C6-MMC was set at 36.8 µM. The A549 cells were seeded into a 96 well plate at 3×103 cells/well in 200 µL of DMEM and incubated under 5% CO2 at 37°C for 24 h. Then, 200 µL of fresh medium containing SWCNTs-CWKG(KWKG)6-(PEG)12 at 6.25–50 µg/mL or SWCNTs-CWKG(KWKG)6-(PEG)12-C6-MMC at 50 µg/mL of SWCNTs concentration was added, and the plate was incubated for 12–48 h. Following the incubation periods, the cells were washed with phosphate buffer solution (PBS) three times. Then, 200 µL of fresh DMEM and 20 µL of WST-1 assay solution were added and the resultant mixture was incubated for 4 h. After the incubation, the absorbance of each well of a 96 well plate was measured at 430 nm with the Eon Microplate Spectrophotometer (BioTek, Winooski, VT, U.S.A.). The cell viability was calculated by utilizing the following equation where Abssample, Abscontrol, and Absblank represent the absorbance of the SWCNTs composite treated cells, untreated cells, and the medium, respectively.
 |
In the pegylation of SWCNTs-CWKG(KWKG)6, modification extents determined with FITC-(PEG)12-NHS were approximately 5.6, 6.9, 9.4, 11.8 and 13.6% of all amino groups for reaction conditions at 10–50% of FITC-(PEG)12-NHS concentrations. This corresponded to 0.8, 1.0, 1.3, 1.7 and 1.9 PEG molecules per one CWKG(KWKG)6 peptide, respectively (Fig. 5). The pegylated SWCNTs-CWKG(KWKG)6-(PEG)12 exhibited superior dispersion stability in an aqueous solution and cell culture medium for 24 h by visual observation, like SWCNTs-(KWKG)7-(PEG)12 complex reported previously17) (data not shown).

FITC-(PEG)12-NHS was added to SWCNTs-CWKG(KWKG)6 dispersion with the concentrations set at 10–50% against all the amino groups of SWCNTs-CWKG(KWKG)6. The results indicated the PEG modification extents of amino groups and the numbers of PEG molecules conjugated to a CWKG(KWKG)6 peptide molecule with the mean±S.D. (n=3).
The aziridine group of MMC was transformed with EMCA by EDC reaction for affording a linker with the maleimide group necessary for conjugation to the thiol group of SWCNTs-CWKG(KWKG)6-(PEG)12. The concentration of the synthesized MMC-EMCA was determined with 4-PDS because the absorbance of MMC and maleimide group partially overlapped approximately at 364 nm. The difference between the MMC-EMCA concentration measured with molar extinction coefficient and MMC-EMCA concentration measured with 4-PDS was as low as approximately 4.5%. Thus, this method could be used for the concentration measurement of the maleimide group.
Synthesis of SWCNTs-CWKG(KWKG)6-(PEG)12-C6-MMC and Determination of MMC ContentMMC-EMCA was easily conjugated to SWCNTs-CWKG(KWKG)6-(PEG)12 with the reaction between maleimide group and thiol group in neutral buffer. However, the conjugated MMC could be liberated due to the hydrolysis especially at the basic pH conditions. Hence, the MMC conjugation was performed in a pH 6.5 MES buffer.
As shown in Fig. 6, almost all of the thiol groups of SWCNTs-CWKG(KWKG)6-(PEG)12 were modified with MMC-EMCA to SWCNTs-CWKG(KWKG)6-(PEG)12-C6-MMC regardless of the PEG modification extents. Thus, PEG modification did not affect the conjugation of MMC-EMCA probably because the sufficient amount of MMC-EMCA, i.e., five holds against thiol groups was applied. In the following experiments, SWCNTs-CWKG(KWKG)6-(PEG)12-C6-MMC with 13.6% PEG modification extent was employed.

The MMC conjugation extent was calculated with absorbance at 364 nm and the MMC molar extinction coefficient. The percentages show MMC conjugation extent against all the thiol groups of SWCNTs-CWKG(KWKG)6-(PEG)12 with the mean±S.D. (n=3).
Figure 7 shows the release of MMC from the dispersion of SWCNTs-CWKG(KWKG)6-(PEG)12-C6-MMC in the buffer solution in the pH range of 5.0–8.0, wherein the release kinetics followed a first order equation, and the release rate was accelerated with an increase in pH. The release half-lives (T1/2) of MMC from SWCNTs-CWKG(KWKG)6-(PEG)12-C6-MMC was approximately 32.2 h at pH 8.0, whereas it was prolonged to 256.2 h at pH 5.0 (Table 1). The released compound was checked with a reverse phase HPLC after incubation in pH 8.0 isotonic HEPES buffer at 37°C for 5 d. It was confirmed to obtain the same retention time with free MMC. This suggested the prolonged release of MMC itself via pH dependent hydrolysis.

SWCNTs-CWKG(KWKG)6-(PEG)12-C6-MMC with 13.6% PEG modification extent was tested. The released MMC was determined with an absorbance at 364 nm after centrifugal filtration of the SWCNTs composite at various incubation time periods at 37°C in various pH isotonic buffers. The percentages of the remaining MMC are represented by the logarithmic indication.
| pH | T1/2 (h) |
|---|---|
| 5.0 | 256.2 |
| 6.0 | 151.4 |
| 7.0 | 63.5 |
| 8.0 | 32.2 |
The cytotoxicity of SWCNTs-CWKG(KWKG)6-(PEG)12, a carrier wrapped by the pegylated polycationic peptide, was evaluated against A549 cells at various concentrations. At a maximum dose of 50 µg/mL and incubation times of 24 and 48 h, approximately 90 and 75% of the cells survived, respectively (Fig. 8). The cell viability increased depending on the decrease of the SWCNTs-CWKG(KWKG)6-(PEG)12 dose. The cell viability at 25 µg/mL and 48 h incubation was approximately 85%, suggesting a relatively low cytotoxicity of SWCNTs-CWKG(KWKG)6-(PEG)12.

SWCNTs concentration was fixed as 6.25–50 µg/mL and the evaluation was performed with WST-1 assay after various incubation time periods.
The antitumor activity of SWCNTs-CWKG(KWKG)6-(PEG)12-C6-MMC was also evaluated with A549 cells at a dose of 36.8 µM of MMC (50 µg/mL for the composite). SWCNTs-CWKG(KWKG)6-(PEG)12-C6-MMC composite was not aggregated in the culture medium on cell layer until WST-1 assay, suggesting excellent dispersion stability of the pegylated SWCNTs composite under biological condition. The results shown in Fig. 9 indicated that the antitumor activity was considerably inferior to the free MMC during the same time period. This reflected the retarded release of the free MMC from the composite. However, the MMC composite presented significantly stronger cell growth inhibition activity than that of SWCNTs-CWKG(KWKG)6-(PEG)12 as shown in Fig. 8. This suggested the effect of released MMC from SWCNTs-CWKG(KWKG)6-(PEG)12-C6-MMC, a prodrug of MMC.

The MMC and SWCNTs composition concentration was set at 36.8 µM and 50 µg/mL, respectively. The effect of free MMC was also compared at the same concentration. The evaluation was performed with WST-1 assay at various incubation time periods.
In previous studies,11,23) a novel composite material was developed using SWCNTs and peptides such as (KF)14, (EFEA)7, (KFKA)7, (KFCA)7, and (KFKA)6KFCA, which were designed to form a β-sheet structure suitable for wrapping the SWCNTs. The formation of the SWCNTs-peptide composite was confirmed by its performance in water, AFM, transmission electron microscopy observation, and molecular modeling.11) The SWCNTs-peptide composite showed good dispersion stability in aqueous media. The possibility of introducing various functions to the SWCNTs-peptide composite was further demonstrated by several methods, including the introduction of special amino acids, chemical modification, and additional complex formation based on such as electrostatic interaction with DNA. The results suggested the potential of the SWCNTs-peptide composite as a molecular platform on which a desirable structure and/or function could be constructed for biomedical and industrial applications. In our series of investigation, (KWKG)7 was also designed as a cationic peptide suitable for the functionalization of SWCNTs.17) The SWCNTs-(KWKG)7 composite indicated a good dispersion stability in water but was still unstable in cell culture media or other buffered solutions and resulted in aggregation. However, the cellular uptake of pegylated SWCNTs-(KWKG)7-(PEG)12 was advanced depending on improvement of dispersion stability in accordance with the extent of PEG modification.17) Therefore, the PEG modification was adopted to improve the dispersion instability of the SWCNTs-peptide composite while maintaining its unique characteristics and advantages for a wide range of applications for such as DDS carrier.
In this study, SWCNTs-CWKG(KWKG)6-(PEG)12 containing amino groups of lysine residue and thiol group of cysteine residue as functional groups was adopted to prepare a molecular depot or a nano-scale prodrug of MMC. The pegylation of SWCNTs-CWKG(KWKG)6 proceeded in a manner similar to the case of SWCNTs-(KWKG)7 with a modification extent of about 5.6–13.6% (Fig. 5). The obtained SWCNTs-CWKG(KWKG)6-(PEG)12 exhibited good dispersion stability even under the physiological conditions. Thus, the difference of an amino acid between CWKG(KWKG)6 and (KWKG)7 did not significantly affect the PEG modification efficiency and/or the physical or chemical properties. Since the cellular uptake of SWCNTs-(KWKG)7-(PEG)12 was verified,17) cellular delivery of SWCNTs-CWKG(KWKG)6-(PEG)12 was also expected, suggesting its potential as a DDS carrier. The present development of a sustained release device for MMC utilizing the SWCNTs-CWKG(KWKG)6-(PEG)12 is a prototype research for the expansion of the possibility of SWCNTs-peptide-PEG composites as well as the extension of previous studies that focused on macromolecular prodrugs of MMC.8–10)
With respect to the introduction of MMC, the conjugation of MMC-EMCA to the thiol groups in SWCNTs-CWKG(KWKG)6-(PEG)12 was not affected by the PEG modification extent and almost all the thiol groups were modified, as shown in Fig. 6. In general, the PEG modification may inhibit chemical reaction due to a steric obstacle. For example, the ninhydrin reaction of SWCNTs-(KWKG)7 was influenced by pegylation and resulted in a quantitatively unreasonable result (data not shown). However, the thiol group was located in the N-terminal of CWKG(KWKG)6 and the applied MMC-EMCA concentration was five times excess to that of thiol groups in the present reaction. The reaction between the thiol group and the maleimide group was performed in a neutral buffer solution; therefore, MMC-EMCA was conjugated to peptides in pH 6.5 MES buffer to inhibit the release of the MMC from the conjugate. In SWCNTs-CWKG(KWKG)6-(PEG)12-C6-MMC, MMC-EMCA is conjugated to one peptide molecule consisted with 28 amino acid. Due to this composition, introduction of MMC does not influence the dispersibility so much.
The pH dependent first-order release kinetics was observed (Fig. 7) on the release of MMC from SWCNTs-CWKG(KWKG)6-(PEG)12-C6-MMC. This was similar to the results of previous studies on the conjugates with dextran8–10) and poly amino acids24) via various linker structures. In the conjugates, it was verified that the electric charge and the length of the linker affected the release of MMC.9,25) The release characteristics of the SWCNTs-CWKG(KWKG)6-(PEG)12-C6-MMC agreed with these findings, despite a delay of T1/2 equal to 32.2 h in pH 8.0 (Fig. 7). The release of MMC from the MMC-dextran conjugate was proved to proceed by chemical hydrolysis based on probably the neighboring group participation effect and plasma and liver homogenate did not accelerate it.26) Hence, the release control of MMC could be achieved by designing a chemical structure around the amide bond next to the aziridine ring and a linker.
The viability of cells treated by SWCNTs-CWKG(KWKG)6-(PEG)12 composite at 50 µg/mL for 24 and 48 h incubation was approximately 90 and 75%, respectively (Fig. 8). In contrast, SWCNTs-(KWKG)7 without PEG modification showed high cytotoxicity and the cell viability at 50 µg/mL for 24 h incubation indicated complete cell death as reported in a previous study.17) The high toxicity of SWCNTs-(KWKG)7 appeared to be provoked by the polycationic nature of SWCNTs-(KWKG)7 and the SWCNTs aggregates. The SWCNTs-CWKG(KWKG)6-(PEG)12 composite demonstrated reduced cytotoxicity due to suppression of the direct interaction with cell surface and dispersion stability improvements in the cell culture medium caused by pegylation. The antitumor activity of SWCNTs-CWKG(KWKG)6-(PEG)12-C6-MMC indicated a lower growth inhibition activity compared with the free MMC of the same concentration (Fig. 9) probably reflecting release of free MMC.
The antitumor activity of MMC appears after reduction with enzyme in cells. Here, the position 1a and 10 of MMC are alkylating sites, and the activity is clearly reduced by the chemical modification of aziridine group.27) Therefore, the activity of SWCNTs-CWKG(KWKG)6-(PEG)12-C6-MMC should be observed after the release of MMC reflecting the nature of prodrug. The resulted cell viability with free MMC at 12 h showed relatively high variation suggesting crucial condition of cell damage at this time point. Based on these results it is concluded that the growth inhibition activity of SWCNTs-CWKG(KWKG)6-(PEG)12-C6-MMC represented a sustained release of MMC but not a cytotoxicity of SWCNTs-CWKG(KWKG)6-(PEG)12 or its MMC conjugate in the aggregated form.
Drug conjugates with SWCNTs-pegylated peptide composites such as SWCNTs-CWKG(KWKG)6-(PEG)12-C6-MMC could be used in vivo as a controlled release as well as a targeting carrier with unique pharmacokinetic characteristics. This is because the pegylated SWCNTs-peptide composite has superior dispersion stability under physiological conditions. SWCNTs-(KWKG)7-(PEG)12-C6-MMC administered via a systemic route would be trapped by the reticuloendothelial system (RES) after intravenous administration. In addition, it could be rapidly eliminated due to electrostatic interaction with the glomerulus capillary wall. Consequently, the in vivo delivery of SWCNTs-(KWKG)7-(PEG)12-C6-MMC via blood circulation might be inefficient and its application should mostly be demonstrated by local administration, which brings topical sustained release as well as lymphatic delivery. However, the SWCNTs composite with anionic peptide would result in blood retention due to delay of transition to organ and the estimated length of 200 nm for SWCNTs-(KWKG)7-(PEG)1211) suggests further possibility of accumulation in the tumor or inflammation sites due to EPR effect16) like other DDS carrier depending on its electric charge, etc. Therefore, in addition to short length, good dispersibility, and reduced cytotoxicity, several other advantageous properties for DDS could be introduced to SWCNTs-peptide composites through various chemical modifications of the peptide structure. The present study reveals the utility of SWCNTs-(KWKG)7-(PEG)12-C6-MMC as a prodrug of MMC based on SWCNT technology. Moreover, it also indicates the potential of SWCNTs-peptide with pegylation composites as a platform for novel DDS development.
The study developed a novel DDS carrier for the sustained release of anticancer agents employing a composite of SWCNTs and pegylated CWKG(KWKG)6 peptides. The conjugate of MMC and SWCNTs-CWKG(KWKG)6-(PEG)12 demonstrated prolonged release of MMC that followed first-order kinetics and was accelerated by a pH increase with the release half-life of 32 h at pH 8.0. In accordance with these findings, the conjugate exhibited delayed but relatively corresponding antitumor activity with free MMC against A549 cell in vitro, although the carrier itself only displayed a slight cytotoxicity. The SWCNTs composite and the artificially designed peptide with pegylation demonstrated excellent dispersion stability in aqueous media and also had wide potential for additional functionalization. Hence, their application of these materials in biomedical fields especially as DDS carriers is extremely promising.
A part of this research was supported by a Giant-in-Aid for Scientific Research (No. 25870366) from the Japan Society for the Promotion of Science.
The authors declare no conflict of interest.