2016 年 39 巻 11 号 p. 1876-1880
2016 年 39 巻 11 号 p. 1876-1880
Genome editing has undergone rapid development during the last three years. It is anticipated that genetically modified organisms (GMOs) for food purposes will be widely produced using the clustered regularly interspaced short palindromic repeat/Cas9 (CRISPR)/Cas9 system in the near future. However, the Cas9 gene may then enter the genomes of GMOs for food if the breeding process is not strictly managed, which could lead to the Cas9 protein or associated peptides being produced within these organisms. A variety of peptides could theoretically be produced from the Cas9 gene by using open reading frames different from that of Cas9 in the GMOs. In this study, Cas9 and the peptides potentially encoded by Cas9 genes were studied regarding their immunogenicity, in terms of the digestibility of Cas9 and the homology of the peptides to food allergens. First, the digestibility and thermal stability of Cas9 were studied. Digestibility was tested with natural or heat-denatured Cas9 in simulated gastric fluid in vitro. The two types of Cas9 were digested rapidly. Cas9 was also gradually degraded during heat treatment. Second, the peptides potentially encoded by Cas9 genes were examined for their homology to food allergens. Specifically, an 8-mer exact match search and a sliding 80-mer window search were performed using allergen databases. One of the peptides was found to have homology with a food allergen.
Food safety is a field of increasing importance, especially in the context of the development of genetically modified organisms (GMOs) for food purposes. Recently, gene editing has been applied to many species, some of which are used to produce agricultural products.1–10) Regarding techniques for gene editing, the clustered regularly interspaced short palindromic repeat/Cas9 (CRISPR)/Cas9 system is a much easier approach than the use of transcription activator-like effector nuclease (TALEN) and zinc finger nuclease (ZFN). This has led to the CRISPR/Cas9 system attracting substantial attention as a new tool for gene editing.3–10) Unfortunately, it is associated with the problem of inducing off-target mutations; however, to the best of our knowledge, no studies have comprehensively investigated GMOs produced by this system for off-target mutations. It is anticipated that this problem will soon be reduced or resolved because the CRISPR/Cas9 system is continually being improved.11,12) There is also the expectation that more GMOs for food purposes will be produced using this system in the future. Against this background, agricultural products originating from these GMOs may contaminate the food supply.
There are three different techniques for gene editing using the CRISPR/Cas9 system. Namely, a plasmid containing the Cas9 gene, Cas9 mRNA, and Cas9 protein can be used. Among these three options, the production of GMOs for food purposes using a plasmid containing the Cas9 gene seems to be advantageous because the protocol for using a plasmid is simpler than those using mRNA or protein. However, if a plasmid is used, there is a possibility of the Cas9 gene being incorporated into the host genome. If cells manipulated by the CRISPR/Cas9 system are not sufficiently screened for the absence of the Cas9 gene in the genome, cells whose genome contains the Cas9 gene may be used to produce GMOs for food purposes. In such cases, Cas9 or its associated peptides may be produced in these GMOs. Various peptides can theoretically be produced from the Cas9 gene using open reading frames (ORFs) different from that of Cas9 in the GMOs. Thus, the immunogenicity of Cas9 and such peptides and their potential allergenicity need to be studied as a particularly important issue in the field of GM food.
As such, in this study, we focused on the digestibility and thermal stability of Cas9. Next, we examined the homology of the above-mentioned peptides to known food allergens. These approaches have also been adopted by the Ministry of Health, Labour and Welfare of Japan, to assess proteins newly introduced into GM food for which the allergenicity is not known.13) To the best of our knowledge, the homology of Cas9 with known food allergens has not been examined yet, so we considered this an important subject for investigation.
pMJ806, an expression plasmid of Cas9, was a gift from Professor Jennifer Doudna (Addgene plasmid #39312).14) Recombinant Cas9 protein was expressed in Escherichia coli using this plasmid and purified, as described by Jinek et al.14) Briefly, it was purified by a combination of Ni-NTA agarose (Qiagen, Hilden, Germany), ion exchange, and gel filtration chromatography.
Purity of Cas9 PreparationThe purity of the Cas9 preparation was examined by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and Coomassie Brilliant Blue (CBB) staining, and using gel filtration by monitoring UV absorption at 280 or 254 nm. The purity of the Cas9 preparation was calculated by the ratios of the peak areas of the chromatogram from gel filtration.
Enzymatic ActivityThe in vitro plasmid cleavage assay was performed in accordance with a previous report.14) Protospacer 2 plasmid DNA described in that report was used as the plasmid with the target sequence. This plasmid contains pUC19 as a backbone, so pUC19 was used· as a negative control of the assay. Guide RNA was synthesized by a chemical method.
Digestion by Simulated Gastric Fluid (SGF)The digestibility of Cas9 protein in SGF was examined, as described by Astwood et al.15) Briefly, 420 ng/µL Cas9 protein was digested in 35-µL aliquots of SGF [0.32% pepsin (Sigma-Aldrich, St. Louis, MO, U.S.A.), 0.03 M NaCl, 0.084 M HCl (pH 1.2)]. Incubation was performed at 37°C and a 7-µL aliquot was taken and mixed with 2.1 µL of 0.2 M Na2CO3 to stop the reaction at the following times: 0, 0.5, 1, 2, and 4 min. These were mixed with equivalent volumes of 2× Laemmli SDS-PAGE sample loading buffer and heated at 100°C for 15 s. To examine the effect of preheating on digestibility, Cas9 protein solution was preheated at 100°C for 5 min and immediately chilled. Then, SGF was added.
Thermal Stability of Cas9To test the thermal stability of Cas9, 720 ng/µL Cas9 protein was heated at 100°C in 10-µL aliquots for the following times: 0, 0.5, 1, 2, and 5 min, and then immediately chilled. These samples were then mixed with equivalent volumes of 2× Laemmli SDS-PAGE sample loading buffer and heated at 100°C for 15 s.
SDS-PAGE and Western BlottingProtein samples were electrophoresed on a 10% SDS-polyacrylamide gel and transferred to a polyvinylidene difluoride membrane. The membranes were blocked in Blocking One (Nacalai Tesque, Kyoto, Japan) and then incubated with diluted Guide-it™ Cas9 Polyclonal Antibody (TaKaRa Bio USA, Mountain View, CA, U.S.A.). After washing, the membranes were incubated with diluted alkaline phosphatase-conjugated goat anti-rabbit IgG and washed again. The membranes were then stained with Alkaline Phosphatase Conjugate Substrate Kit (Bio-Rad, Hercules, CA, U.S.A.).
Homology Search of Peptides Potentially Encoded by the Cas9 GenesNucleotide sequences of Cas9 genes codon-optimized for human, corn, or soybean were obtained from Addgene.16–18) Each of them was translated into amino acids with six ORFs using the sense strand or the anti-sense strand. Peptides longer than 19 amino acid residues were selected for the following homology searches with known food allergens. Peptides without initiation codons were included in our searches. The Allergen Database19) was searched for 8-mer exact matches of these peptides. The Structural Database of Allergenic Proteins20) was used for a sliding 80-mer window search of these peptides. We considered the result to be positive when homology was greater than 35%.
Recombinant Cas9 was expressed in E. coli and purified. It was then analyzed for its purity. Cas9 was detected as a single band when it was analyzed by CBB staining. This indicated that no other proteins contaminated the Cas9 preparation. However, when the Cas9 preparation was examined by gel filtration, a substance with a lower molecular weight than Cas9 was detected. The purity of Cas9 was 89.2 or 82.5% when the Cas9 preparation was analyzed by gel filtration and UV absorption at 280 or 254 nm, respectively.
Enzymatic ActivityRecombinant Cas9 was analyzed for its catalytic activity by the in vitro plasmid cleavage assay. The digested, linearized plasmid was clearly detected in the assay using protospacer 2 plasmid DNA that contained the target sequence. However, it was not detected in that of pUC19 in a control experiment. The results are shown in Fig. 1, which indicate that the cleavage by Cas9 is specific. We thus confirmed that the Cas9 we prepared was catalytically active.

The plasmids were incubated with Cas9 and gRNA for the indicated periods (min). Supercoiled plasmid and linear cleavage product were separated by 0.8% agarose gel electrophoresis. MW stands for DNA molecular weight markers (λ-HindIII).
Recombinant Cas9 expressed in E. coli was used in the experiment. Cas9 (ca. 160 kDa) was not detected at all at 0.5 min, which means that it was completely digested within this period. Its cleaved fragments were also not detected. The digestion pattern of Cas9 is shown in Fig. 2. Cas9 was not detected even when polyclonal antibody was used in Western blotting (Fig. 2, panel B). This also suggests that Cas9 was completely digested. When Cas9 was incubated in SGF for longer periods up to 32 min, no degraded fragments were detected (Fig. 3).
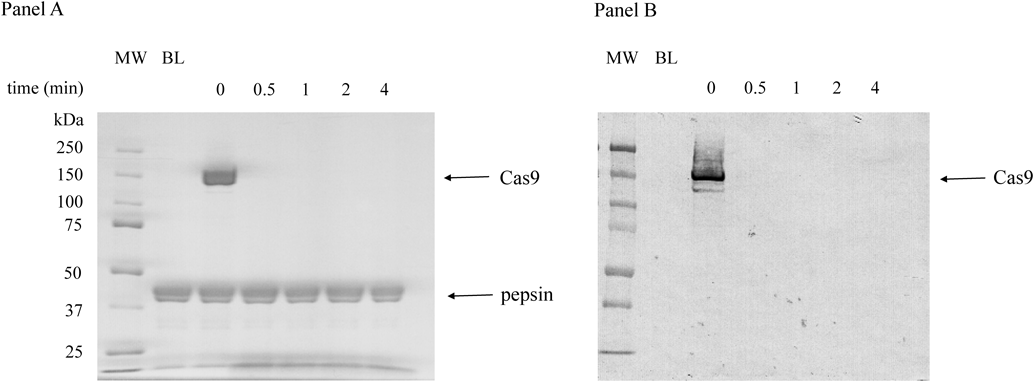
The patterns of Coomassie Brilliant Blue staining and Western blotting are shown in Panels A and B.

The patterns of Coomassie Brilliant Blue staining and Western blotting are shown in Panels A and B.
The thermal stability of Cas9 was examined at 100°C for up to 5 min. The band of Cas9 gradually decreased in intensity depending on the duration of heat treatment in Western blotting. This suggests that Cas9 is not stable upon exposure to heat. The cleaved fragments were not detected. A typical pattern of Western blotting is shown in Fig. 4.

Cas9 was heated at 100°C for the indicated periods (min). The pattern of Western blotting is shown.
The possibility that heat-treated Cas9 is resistant to digestion was studied. Cas9 was heated at 100°C for 5 min and then incubated in SGF. The digestibility of Cas9 under these conditions was tested. A low level of Cas9 was detected at 0 min without incubation in SGF. This indicates that a large proportion of Cas9 was degraded during the heat treatment. The remaining Cas9 was digested completely within 0.5 min in SGF. The digestion pattern of Cas9 under these conditions is shown in Fig. 5.

Cas9 was preheated at 100°C for 5 min and digested by SGF for the indicated periods (min). The pattern of Western blotting is shown. The rightmost lane is a control to confirm that Cas9 was abundant in the solution without preheating or digestion.
No homology between Cas9 and known food allergens was identified when an 8-mer exact match search and a sliding 80-mer window search were performed. Large numbers of potential peptides were obtained from Cas9 genes using ORFs different from that of Cas9. These potential peptides were examined for homology with known food allergens. The results of the homology search are shown in Table 1. For Cas9 genes codon-optimized for human or corn, no peptides showed matches in the 8-mer exact match search or the sliding 80-mer window search. For the Cas9 gene codon-optimized for soybean, no matches were found in the sliding 80-mer window search, but one match was found in the 8-mer exact match search. The potential peptide (RSAVVSSSSSSSSPPPRMARYRTFLCSYRI) shows an 8-mer exact match with serine carboxypeptidase 2 in common wheat.
| Codon-optimized Cas9 gene | Number of potential peptides | 8-Mer exact match | Sliding 80-mer window search |
|---|---|---|---|
| For human | 92 | Negative | Negative |
| For corn | 116 | Negative | Negative |
| For soybean | 94 | Positive* | Negative |
* A potential peptide encoded by the anti-sense strand has an 8-mer exact match with serine carboxypeptidase 2 in Triticum aestivum. The sequence of the peptide is RSAVVSSSSSSSSPPPRMARYRTFLCSYRI. The underline indicates the matching region.
In this study, we considered the potential effects of incorporation of the Cas9 gene into GMOs for food use. We describe a problematic area regarding the breeding of GM plants in particular. Agrobacterium has been frequently used to produce GM plants. When a GM plant is bred using the CRISPR/Cas9 system and Agrobacterium, the Cas9 gene can be incorporated into the plant genome. If backcrossing is performed, the clones without the Cas9 gene in their genomes will be selected. However, if the step of backcrossing is omitted, the Cas9 gene would be retained in the plant genome. When a GM plant for food purposes is produced in such a way, it may thus have the Cas9 gene in its genome. This would result in the general public eating food containing Cas9 or associated peptides, despite the Cas9 gene not having been incorporated into the GMO intentionally. Given this eventuality, we studied the safety of Cas9 and associated peptides in GM food.
First, we studied the digestibility of Cas9 in the stomach. We focused on this issue because food allergens are reported to exhibit sufficient gastric stability to reach the intestinal mucosa, where absorption and sensitization can occur, and because stability against digestion is a significant parameter that distinguishes food allergens from nonallergens. To assess the digestibility of proteins newly introduced into GM crops in the stomach, an in vitro digestion experiment with SGF was developed15) and has been used widely.21–23) We also used this experiment in our study.
Cas9 was digested rapidly in SGF, even though Cas9 was used at a high concentration in our experiment. This rapid digestibility suggests that Cas9 would be degraded in the stomach if it were ingested as a food component, and that the possibility of food allergenicity resulting from the consumption of this protein should be extremely low. We do not think that studies of the digestibility of Cas9 in the bowels and the ability of Cas9 to bind immunoglobulin E (IgE) are necessary because it is expected to be digested rapidly in the stomach and thus should not enter the bloodstream. Next, we examined the thermal stability of Cas9 and demonstrated that it is not stable upon exposure to heat.
We considered the case of processed food being made using GMOs containing Cas9. Processed food is often heated during its production. We thus considered the possibility that Cas9 aggregates and becomes resistant to digestion by SGF during heat treatment. If this were to happen, Cas9 could be antigenic or toxic. However, we demonstrated that Cas9 was degraded rather than aggregated during heat treatment and that a small amount of residual Cas9 was rapidly digested by SGF. Complete disappearance of Cas9 in these conditions suggests that the heat treatment of Cas9 is not harmful. Taking these findings together, Cas9 did not exhibit immunogenicity leading to allergenicity.
Next, the potential peptides encoded by the Cas9 genes were studied for homology with known food allergens by a bioinformatics approach. These peptides can be produced when fragments of Cas9 gene are incorporated into ORFs of endogenous genes of host genome. The peptides potentially encoded by Cas9 genes codon-optimized for human or corn did not match the sequences of known food allergens. They are thus thought to be safe because an allergenic peptide would be expected to have homology with known allergens. However, a peptide encoded by the Cas9 gene codon-optimized for soybean was shown to have one site homologous to serine carboxypeptide 2 in common wheat when an 8-mer exact match search was performed. Epitopes of this allergen are currently unknown. Thus, conclusions cannot be drawn regarding whether the exactly matching 8-mer sequence (SSSSSSSS) is immunogenic or allergic. An assessment of the safety of this peptide is a subject for future investigation.
We used the sequences of the Cas9 gene codon-optimized for human, corn, or soybean rather than the original Streptococcus pyrogenes Cas9 gene sequence as queries for our homology search. The Cas9 gene codon-optimized for human is widely used for genome editing in mammalian livestock. This gene was also used to generate transgenic chickens.5) Regarding the use of the Cas9 gene in plants, we selected Cas9 genes codon-optimized for corn or soybean in this study. The status of corn and soybean as major crops and the fact that these Cas9 genes have been deposited in Addgene mean that these resources are available and potentially beneficial to many producers of GMOs. It is thus anticipated that these genes will be used widely in the future. In our analyses in the present study, we assumed the possibility that a fragment of the Cas9 gene could be incorporated into a host genome. We also assumed the possibility that the Cas9 gene could be incorporated into it in the opposite direction. In addition, we took into account the possibility that the Cas9 gene could be introduced into the ORF of an endogenous gene and that the peptides encoded by this gene would be expressed as fusion proteins. As such, peptides without initiation codons were included in the analysis in this study. Prediction of allergenicity of peptides potentially encoded by a transgene is difficult and problems still exist. Experts are discussing about it to make an international agreement.24) Thus, it is not appropriate to discuss probability of these peptides inducing allergy in detail at the present time. On the other hand, the Japanese authorities adopt an 8-mer exact match search and a sliding 80-mer window search with known food allergens to predict the allergenicity of peptides encoded by a transgene newly introduced into GMOs. Here, we used the same criteria for the examination of peptides potentially encoded by Cas9 genes codon-optimized for human, corn, or soybean. As such, this study should contribute to the safety of food.
Cas9 did not exhibit immunogenicity leading to allergenicity in our in vitro experiments. The results of searches for homology between peptides potentially encoded by Cas9 genes codon-optimized for human or corn and known food allergens were negative. However, in a homology search of the peptides potentially encoded by the Cas9 gene codon-optimized for soybean, one peptide exhibiting homology with a food allergen was found.
This work was supported by a Health Labour Sciences Research Grant (H25-shokuhin-ippan 015).
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.
Supplementary Figures Elution profiles of the gel filtration of Cas9 preparation with UV absorption monitor at 280 and 254 nm are shown in Panels A and B, respectively.