Abstract
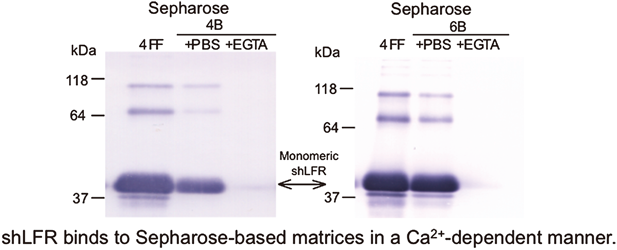
A soluble form of human intestinal lactoferrin receptor (shLFR) is identical to human intelectin-1 (hITLN-1), a galactofuranose-binding protein that acts as a host defense against invading pathogenic microorganisms. We found that recombinant shLFR, expressed in mammalian cells (CHO DG44, COS-1, and RK13), binds tightly to Sepharose 4 Fast Flow (FF)-based matrices in a Ca2+-dependent manner. This binding of shLFR to Sepharose 4 FF-based matrices was inhibited by excess D-galactose, but not by D-glucose, suggesting that shLFR recognizes repeating units of α-1,6-linked D-galactose in Sepharose 4 FF. Furthermore, shLFR could bind to both Sepharose 4B- and Sepharose 6B-based matrices that were not crosslinked in a similar manner as to Sepharose 4 FF-based matrices. Therefore, shLFR (hITLN-1) binds to Sepharose-based matrices in a Ca2+-dependent manner. This binding property is most likely related to the ability, as host defense lectins, to recognize sepharose (agarobiose)-like structures present on the surface of invading pathogenic microorganisms.