Abstract
Pain is sensed, transmitted, and modified by a variety of mediators and receptors. Histamine is a well-known mediator of pain. In addition to their anti-histaminic effects, the classical, or 1st generation, anti-histamines (1st AHs) possess, to various degrees, anti-muscarinic, anti-serotonergic, anti-adrenergic, and other pharmacologic effects. Although there have been attempts to use 1st AHs as analgesics and/or analgesic adjuvants, the advent of non-steroidal anti-inflammatory drugs (NSAIDs) discouraged such trials. We previously reported that in patients with temporomandibular disorders, osteoporosis, and/or osteoarthritis, the analgesic effects of certain 1st AHs (chlorpheniramine and diphenhydramine) are superior to those of the NSAIDs flurbiprofen and indomethacin. Here, we compared analgesic effects among 1st AHs and NSAIDs against responses shown by mice to intraperitoneally injected 0.7% acetic acid. Since 1st AHs are water soluble, we selected water-soluble NSAIDs. For direct comparison, drugs were intravenously injected 30 min before the above tests. Histamine-H1-receptor-deficient (H1R-KO) mice were used for evaluating H1-receptor-independent effects. The tested 1st AHs (especially cyproheptadine) displayed or tended to display analgesic effects comparable to those of NSAIDs in normal and H1R-KO mice. Our data suggest that the anti-serotonergic and/or anti-adrenergic effects of 1st AHs make important contributions to their analgesic effects. Moreover, combination of a 1st AH with an NSAID (cyclooxygenase-1 inhibitor) produced remarkably potent analgesic effects. We propose that a 1st AH, by itself or in combination with a cyclooxygenase-1 inhibitor, should undergo testing to evaluate its usefulness in analgesia.
Histamine is a well-known mediator of pain and/or itch.1) Consequently, many attempts have been made to use classical, or 1st generation, anti-histamines (1st AHs) as analgesics and/or analgesic adjuvants.2–4) However, the advent of various types of non-steroidal anti-inflammatory drugs (NSAIDs), and their rapid spread, seems to have discouraged such efforts to promote 1st AHs as analgesics.
Pain in both masticatory muscles and temporomandibular joints is a major sign of temporomandibular disorders (TMD), which are believed to result from strenuous, improper, or abnormal occlusion, including bruxism and/or prolonged clenching. Interestingly, Watanabe et al. noted that the analgesic effect of chlorpheniramine (a 1st AH) is superior to that of flurbiprofen (an NSAID) in patients with TMD.5) Bone-joint-muscle pain is commonly experienced by elderly patients with osteoporosis (OP) and/or osteoarthritis (OA). Recently, Fujita et al. reported that the analgesic effect of diphenhydramine (a 1st AH) is superior to that of indomethacin (an NSAID) in patients with OP and/or OA.6) It is notable that muscle fatigue and pain are common factors among TMD, OP, and OA, and that histamine has been suggested to be causally involved in muscle fatigue and pain.7–9) Collectively, these reports encourage the view that 1st AHs may indeed exert analgesic effects in patients with TMD, OP, and/or OA.
However, pain is transmitted, sensed, and modified via a variety of mediators other than histamine and its receptors. These mediators include serotonin, noradrenaline, bradykinin, and prostaglandins. In addition to their antagonistic effects against the histamine-H1-receptor (H1R), 1st AHs possess, to various degrees, anti-muscarinic, anti-serotonergic, anti-adrenergic, and other pharmacologic actions.2,10,11) For example, upon local application, diphenhydramine (a typical 1st AH) is reportedly as effective at pain prevention as the local anesthetic drug lidocaine.12) Thus, it is very likely that effects other than antagonism toward H1R are involved in the analgesic effects of 1st AHs.
Here, we examined (i) whether 1st AHs do indeed display analgesic effects, and (ii) how antagonism toward receptors other than H1R might contribute to such analgesic effects. Examining the writhing responses of mice to intraperitoneally injected dilute acetic acid is widely used as a method for evaluating the analgesic or pain-augmenting effects of test materials.13,14) Using this method, we compared analgesic effects among 1st AHs and NSAIDs. Since 1st AHs are water-soluble, we selected water-soluble NSAIDs (diclofenac, indomethacin, and meloxicam). For easy and direct comparison, each drug was intravenously injected 30 min before the writhing test. To evaluate the involvement of antagonism toward receptors other than H1R, we used mice deficient in H1R (H1R-KO mice).
MATERIALS AND METHODS
MiceBALB/c and C57BL/6 mice were purchased from CREA (Tokyo, Japan), while C3H/HeN and ddY mice were from SLC (Hamamatsu, Japan). H1R-KO mice (C57BL/6 background) were established as previously described,15) bred in our laboratory, and confirmed by genotyping during this study. The experiments were performed in accordance with International Association for the Study of Pain (IASP) guidelines for the study of pain in animals.16) All experiments complied with the Guidelines for Care and Use of Laboratory Animals in Tohoku University and were approved by the Committee on Animal Research of Tohoku University.
ReagentsAcetic acid was purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). The drugs used in this study are listed in Table 1. All were dissolved in sterile saline, with the pH of the solutions being adjusted to 7 if necessary with NaOH or HCl, and intravenously injected into mice (0.1 mL/10 g body weight). Experimental protocols and doses are described in the text or the relevant figure legend.
Table 1. Drugs Tested in This Study
| Name (abbreviation and molecular weight) | Note |
|---|
| 1st AHs |
| Diphenhydramine hydrochloride (Diph, 291)§ | An ethanolamine [sedative (relatively strong), anti-motion sickness, local anesthetic, anti-cholinergic] |
| S-(+)-Chlorpheniramine maleate (Chlor, 391)* | An alkylamine [sedative (relatively weak)] |
| Promethazine hydrochloride (Prom, 321)¶ | A phenothiazine [local anesthetic, anti-motion-sickness, anti-cholinergic, anti-adrenergic] |
| Cyproheptadine hydrochloride (Cypro, 351)* | A piperidine [anti-serotonergic (strong 5HT2A receptor antagonist), anti-motion sickness] |
| NSAIDs |
| Diclofenac sodium (Diclo, 318)¶ | A phenylacetic acid [cyclooxygenase (Cox)-1 inhibitor] |
| Meloxicam sodium hydrate (Melo, 373)* | An oxicam [Cox-2 inhibition>Cox-1 inhibition] |
| Indomethacin (Indo, 358)¶ | An indoleacetic acid [Cox-1 inhibitor] |
| Anti-cholinergic drug |
| (−)-Scopolamine hydrochloride (Scop, 340)* | Anti-motion sickness; muscarinic acetylcholine receptor antagonist |
| Anti-serotonergic drugs |
| Ketanserin tartrate (Keta, 395)* | 5HT2A receptor antagonist |
| Granisetron hydrochloride (Grani, 348)* | 5HT3 receptor antagonist |
| Anti-adrenergic drugs |
| Prazosin hydrochloride (Prazo, 420)¶ | α1 Receptor antagonist |
| Yohimbine hydrochloride (Yohim, 391)¶ | α2 Receptor antagonist |
* From Sigma (St. Louis, MO, U.S.A.), § from Kowa Company, Ltd. (Nagoya, Japan), ¶ from Wako Pure Chemical Industries, Ltd. (Osaka, Japan).
Writhing (abdominal constriction) was induced by intraperitoneal injection of 0.7% (v/v) acetic acid (0.1 mL/10 g body weight). The number of writhing movements (“writhings”) was counted in the period from 5 to 30 min after the acetic acid injection.
Data AnalysisExperimental values are each given as the mean±standard deviation (S.D.). The statistical significance of differences was evaluated using a Bonferroni multiple-comparison test after an ANOVA, p values less than 0.05 being considered significant.
RESULTS
H1R-KO and C3H/HeN Mice Are Useful for the Writhing TestThe nociceptive responses of H1R−/− mice are reportedly lower than those of H1R+/+ mice in various tests, including the writhing test.17) However, in the present study, the writhing responses of male and female H1R-KO mice were not different from those shown by male and female C57BL/6N mice (nor from those of male or female BALB or male C3H/HeN mice) (Fig. 1A). We suppose that this may be due to the fact that wild-type C57BL/6 strain used in the present study was commercially obtained, but not derived from the strain used in the original study.17) In addition, the following factors may also be involved. (i) The sensitivities to nociceptive stimuli and/or to analgesic drugs are reportedly different among strains of mice.18) (ii) It has also been reported that complete inactivation of a gene may result in altered expressions of related genes or physiologic compensation for the loss of the gene product.19) Thus, the pain sensitivity of H1R-KO mice might have changed during repeated breeding cycles. In any event, the finding that H1R-KO mice exhibit writhing responses suggests that H1R-KO mice are useful for examining the analgesic effects of 1st AHs that are not mediated via H1R. Also note that there was no significant gender difference in H1R-KO mice. Thus, in the following experiments on H1R-KO mice, both males and females were used, these being available to us since they were bred in our laboratory. We confirmed our previous finding20) that the writhing response of female C3H/HeN mice is, or tends to be, greater than those of other strains. Thus, this strain may be convenient for evaluating the analgesic effects of test samples.
Effects of 1st AHs and NSAIDs in C3H/HeN and H1R-KO MiceThe molecular weights of the tested drugs are similar (around 300 to 400) (Table 1). In the experiments shown in Figs. 1B and C, the dose of each drug was 2.5 mg/kg (6–8 µmol/kg). In female C3H/HeN mice, two of the four 1st AHs tested, namely cyproheptadine and chlorpheniramine, exhibited significant analgesic effects (Fig. 1B). In H1R-KO mice, among the three 1st AHs tested only cyproheptadine exhibited a significant analgesic effect (Fig. 1C), although diphenhydramine and promethazine tended to be analgesic. None of the three NSAIDs tested had significant analgesic effects, although they tended to be analgesic (Fig. 1C). These results indicate that (i) some 1st AHs (especially cyproheptadine) do indeed have analgesic effects, and (ii) such analgesia may involve effect(s) other than H1R-mediated anti-histaminergic effects.
Effects of Anti-serotonergic, Anti-cholinergic, and Anti-adrenergic DrugsNotably, cyproheptadine had a powerful analgesic effect in the writhing test in both C3H/HeN and H1R-KO mice (Figs. 1B, C). In both C3H/HeN and H1R-KO mice, ketanserin significantly reduced the writhing response (Figs. 1B, C), although granisetron only tended to be analgesic in these strains. The α1-adrenergic antagonist prazosin was effective, too, although there was no α2-mediated analgesic effect with yohimbine (Fig. 1C). Unlike these antagonists, the muscarinic acetylcholine-receptor antagonist scopolamine was not effective at all (Fig. 1C). These results suggest that anti-serotonergic (especially 5HT2A-blocking) effects and anti-adrenergic (especially α1-blocking) effects may be involved in the analgesic effects shown by 1st AHs.
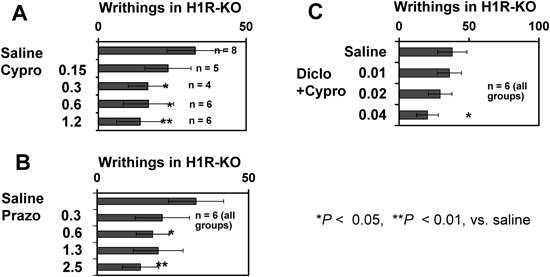
Minimum Effective Doses of Cyproheptadine, Ketanserin, and Prazosin in H1R-KO MiceThe analgesic effect of cyproheptadine was dose-dependent, and was evident even at 0.3 mg/kg in H1R-KO mice (Fig. 2A). However, ketanserin (which was analgesic at 2.5 mg/kg; see above) was not analgesic at 1.2 mg/kg or less (data not shown). An analgesic effect of prazosin was evident even at 0.6 mg/kg (Fig. 2B). These results suggest that (i) inhibition of 5HT2A receptors and inhibition of α1-adrenergic receptors are each effective at producing some degree of analgesia, and (ii) inhibition of 5HT2A receptors may contribute to the analgesic effect of cyproheptadine.
Effects of an NSAID+a 1st AHFinally, we examined the effect of a combination of an NSAID plus a 1st AH in H1R-KO mice (Fig. 1C lower portions). Either cyproheptadine (a 1st AH with anti-5H2A effects) or promethazine (a 1st AH with multiple effects, including anti-adrenergic effects), when combined with the NSAID diclofenac, produced markedly potent analgesic effects, although these effects were not significantly greater than that of cyproheptadine alone. It should be noted that the analgesic effects of diclofenac and promethazine, by themselves, were not significant (Fig. 1C). Cyproheptadine+diclofenac was significantly effective when each was at 0.04 mg/kg (Fig. 2C). However, we observed no augmented effect by combinating dicofenac with either diphenhydramine or meloxicam. These results suggest that a combination of cyproheptadine or promethazine with a cyclooxygenase-1 inhibitor induces a very potent analgesic effect.
DISCUSSION
H1Rs are distributed widely in a variety of cells, including central nervous system and primary sensory neurons.21,22) First AHs have been suggested to have analgesic effects on both somatic and visceral pain.17) In the present study, 1st AHs exhibited analgesic effects in the writhing test in both C3H/HeN and H1R-KO mice.
As described in Introduction and Table 1, 1st AHs have several pharmacological effects other than anti-histaminic effects. All the 1st AHs used in the present study have some degree of anti-serotonergic activity [strong (cyproheptadine), medium (promethazine and diphenhydramine), or weak (chlorpheniramine)].10) The 5HT2A antagonist ketanserin exhibited an analgesic effect in both C3H/HeN and H1R-KO mice (Figs. 1B, C). It has been reported that serotonin may mediate mainly pronociceptive effects in the periphery and both antinociceptive and pronociceptive effects within the central nervous system.23,24) It is well known that both histamine and serotonin are local mediators of pain and inflammation.11) We suggested previously that histamine is locally involved both in muscle fatigue and in musculoskeletal pain in TMD,5,7,9) and serotonin has also been suggested to be involved in TMD as a local pain mediator.25) Our recent clinical study—in which ointments of diphenhydramine and indomethacin were painted over the area where patients with OA and/or OP reported pain sensation—demonstrated an analgesic effect of diphenhydramine that was superior to that of indomethacin.6) Moreover, the pain-relieving effect of oral chlorpheniramine in TMD is greater than that of oral flurbiprofen (an NSAID).5) Collectively, those observations lead us to suppose that the analgesic effects of 1st AHs may be displayed mainly via their local effects, involving blocking effects on peripheral histamine and serotonin receptors.
Adrenergic α1-receptors have been reported to be involved in various nociceptive responses, including writhing in mice,26) ATP receptor-mediated nociception in rats,27) streptozotocin-induced hyperalgesia in mice,28) and thermal hyperalgesia in humans.29) Indeed, the α1-antagonist prazosin exhibited a strong analgesic effect in the present writhing test, too. Thus, the anti-adrenergic effects of 1st AHs may also contribute to their analgesic effects.
Interestingly, a combination of a 1st AH (cyproheptadine or promethazine) with the NSAID diclophenac (cyclooxygenase-1 inhibitor) had the strongest analgesic effect seen in the present study (Fig. 1C). An analgesic effect of combined cyproheptadine+diclophenac was detected when each was at as low a dose as 0.04 mg/kg (Fig. 2C). It was recently reported that prostaglandin E2, which is released from epithelial cells in response to various inflammatory stimuli, stimulates mast cells to release histamine, leading to inflammation.30) Moreover, prostaglandin E2 is released into the peritoneal cavity by dilute acetic acid.31) These findings suggest that concomitantly blocking histaminergic, serotonergic, and adrenergic receptors, together with an inhibition of prostaglandin synthesis, may lead to a particularly powerful analgesic effect.
In conclusion, in mice, some 1st AHs displayed potent analgesic effects comparable to or greater than those of NSAIDs. Anti-histaminergic, anti-serotonergic (especially anti-5HT2A), and anti-adrenergic (especially anti-α1) effects of 1st AHs may be involved in their analgesic effects. We propose that administration of a 1st AH, either by itself or in combination with an NSAID (cyclooxygenase-1 inhibitor), warrants evaluation as a useful analgesic strategy, especially in patients with TMD, OP, and/or OA (see Introduction).
Acknowledgments
We are grateful to Dr. R. Timms (Birmingham, U.K.) for editing the manuscript, and to Prof. Kazuhiko Yanai and Dr. Takeo Yoshikawa (Department of Pharmacology, Tohoku University Graduate School of Medicine, Sendai, Japan) for providing H1R-KO mice. This study was supported by Grants from the Japan Society for the Promotion of Science to M. Watanabe (23390439) and M. Tsuchiya (24390429 and 25670813).
Conflict of Interest
The authors declare no conflict of interest.
REFERENCES
- 1) Tiligada E, Kyriakidis K, Chazot PL, Passani MB. Histamine pharmacology and new CNS drug targets. CNS Neurosci. Ther., 17, 620–628 (2011).
- 2) Rumore MM, Schlichting DA. Clinical efficacy of antihistaminics as analgesics. Pain, 25, 7–22 (1986).
- 3) Raffa RB. Antihistamines as analgesics. J. Clin. Pharm. Ther., 26, 81–85 (2001).
- 4) Farzin D, Asghari L, Nowrouzi M. Rodent antinociception following acute treatment with different histamine receptor agonists and antagonists. Pharmacol. Biochem. Behav., 72, 751–760 (2002).
- 5) Watanabe M, Tabata T, Huh JI, Inai T, Tsuboi A, Sasaki K, Endo Y. Possible involvement of histamine in muscular fatigue in temporomandibular disorders: animal and human studies. J. Dent. Res., 78, 769–775 (1999).
- 6) Fujita T, Ohue M, Fujii Y, Jotoku T, Miyauchi A, Takagi Y, Tsuchiya M, Endo Y. Prompt analgesic effect of antihistaminic diphenhydramine ointment on bone-joint-muscle pain, as assessed by skin impedance. Pharmacology, 92, 158–166 (2013).
- 7) Endo Y, Tabata T, Kuroda H, Tadano T, Matsushima K, Watanabe M. Induction of histidine decarboxylase in skeletal muscle in mice by electrical stimulation, prolonged walking and interleukin-1. J. Physiol., 509, 587–598 (1998).
- 8) Niijima-Yaoita F, Tsuchiya M, Ohtsu H, Yanai K, Sugawara S, Endo Y, Tadano T. Roles of histamine in exercise-induced fatigue: favouring endurance and protecting against exhaustion. Biol. Pharm. Bull., 35, 91–97 (2012).
- 9) Yoneda H, Niijima-Yaoita F, Tsuchiya M, Kumamoto H, Watanabe M, Ohtsu H, Yanai K, Tadano T, Sasaki K, Sugawara S, Endo Y. Roles played by histamine in strenuous or prolonged masseter muscle activity in mice. Clin. Exp. Pharmacol. Physiol., 40, 848–855 (2013).
- 10) Rumore MM, Schlichting DA. Analgesic effects of antihistaminics. Life Sci., 36, 403–416 (1985).
- 11) Garrison JC. Histamine, bradykinin, 5-hydroxytryptamine, and their antagonists. Goodman and Gilman’s the Pharmacological Basis of Therapeutics (Gilman AG, Rall TW, Nies AS, Taylor P eds.) 8th ed., Pergamon Press, New York, pp. 575–599 (1990).
- 12) Apiliogullari S, Keles B, Apiliogullari B, Balasar M, Yilmaz H, Duman A. Comparison of diphenhydramine and lidocaine for prevention of pain after injection of propofol: a double-blind, placebo-controlled, randomized study. Eur. J. Anaesthesiol., 24, 235–238 (2007).
- 13) Vinegar R, Truax JF, Selph JL, Hohnston PR. Antagonism of pain and hyperalgesia. Handbook of Experimental Pharmacology. (Vane JR, Ferreira SH eds.) Vol. 5/II, Springer-Verlag, Berlin, pp. 208–222 (1979).
- 14) Ueno A, Matsumoto H, Naraba H, Ikeda Y, Ushikubi F, Matsuoka T, Narumiya S, Sugimoto Y, Ichikawa A, Oh-ishi S. Major roles of prostanoid receptors IP and EP3 in endotoxin-induced enhancement of pain perception. Biochem. Pharmacol., 62, 157–160 (2001).
- 15) Inoue I, Yanai K, Kitamura D, Taniuchi I, Kobayashi T, Niimura K, Watanabe T, Watanabe T. Impaired locomotor activity and exploratory behavior in mice lacking histamine H1 receptors. Proc. Natl. Acad. Sci. U.S.A., 93, 13316–13320 (1996).
- 16) Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain, 16, 109–110 (1983).
- 17) Mobarakeh JI, Sakurada S, Katsuyama S, Kutsuwa M, Kuramasu A, Lin ZY, Watanabe T, Hashimoto Y, Watanabe T, Yanai K. Role of histamine H1 receptor in pain perception: a study of the receptor gene knockout mice. Eur. J. Pharmacol., 391, 81–89 (2000).
- 18) Mogil JS. The genetic mediation of individual differences in sensitivity to pain and its inhibition. Proc. Natl. Acad. Sci. U.S.A., 96, 7744–7751 (1999).
- 19) Rudmann DG, Durham SK. Utilization of genetically altered animals in the pharmaceutical industry. Toxicol. Pathol., 27, 111–114 (1999).
- 20) Kim S, Seiryu M, Okada H, Kuroishi T, Takano-Yamamoto T, Sugawara S, Endo Y. Analgesic effects of the non-nitrogen-containing bisphosphonates etidronate and clodronate, independent of anti-resorptive effects on bone. Eur. J. Pharmacol., 699, 14–22 (2013).
- 21) Hill SJ, Ganellin CR, Timmerman H, Schwartz JC, Shankley NP, Young JM, Schunack W, Levi R, Haas HL. International Union of Pharmacology. XIII. Classification of histamine receptors. Pharmacol. Rev., 49, 253–278 (1997).
- 22) Kashiba H, Fukui H, Morikawa Y, Senba E. Gene expression of histamine H1 receptor in guinea pig primary sensory neurons: a relationship between H1 receptor mRNA-expressing neurons and peptidergic neurons. Brain Res. Mol. Brain Res., 66, 24–34 (1999).
- 23) Bardin L. The complex roles of serotonin and 5-HT receptors in chronic pain. Behav. Pharmacol., 22 (5 and 6), 390–404 (2011).
- 24) Viguier F, Michot B, Hamon M, Bourgoin S. Multiple roles of serotonin in pain control mechansms: implications of 5-HT7 and other 5-HT receptor types. Eur. J. Pharmacol., 716, 8–16 (2013).
- 25) Gerdle B, Ghafouri B, Ernberg M, Larsson N. Chronic musculoskeletal pain: review of mechanisms and biochemical biomarkers as assessed by the microdialysis technique. J. Pain Res., 7, 313–326 (2014).
- 26) Bezerra MM, Lima V, Girão VCC, Teixeira RC, Graça JRV. Antinociceptive activity of sildenafil and adrenergic agents in the writhing test in mice. Pharmacol. Rep., 60, 339–344 (2008).
- 27) Meisner JG, Waldron JB, Sawynok J. α1-Drenergic receptors augment P2X3 receptor-mediated nociceptive responses in the uninjured state. J. Pain, 8, 556–562 (2007).
- 28) Bujalska M, Araźna M, Makulska-Nowak H, Gumułka SW. α1- and α2-Adrenoceptor antagonists in streptozotocin- and vincristine-induced hyperalgesia. Pharmacol. Rep., 60, 499–507 (2008).
- 29) Drummond PD. α1-Adrenoceptors augment thermal hyperalgesia in mildly burnt skin. Eur. J. Pain, 13, 273–279 (2009).
- 30) Morimoto K, Shirata N, Taketomi Y, Tsuchiya S, Segi-Nishida E, Inazumi T, Kabashima K, Tanaka S, Murakami M, Narumiya S, Sugimoto Y. Prostaglandin E2-EP3 signaling induces inflammatory swelling by mast cell activation. J. Immunol., 192, 1130–1137 (2014).
- 31) Murata T, Ushikubi F, Matsuoka T, Hirata M, Yamasaki A, Sugimoto Y, Ichikawa A, Aze Y, Tanaka T, Yoshida N, Ueno A, Oh-ishi S, Narumiya S. Altered pain perception and inflammatory response in mice lacking prostacyclin receptor. Nature, 388, 678–682 (1997).