2016 年 39 巻 6 号 p. 920-926
2016 年 39 巻 6 号 p. 920-926
Solanum cernuum VE. has been used extensively for the treatment of urinary disorders, gonorrhea and skin infections; cernumidine is a major component of S. cernuum (SC) hydroalcoholic extract. The micronucleus test in V79 cells was used to evaluate the genotoxic and antigenotoxic potential of SC and cernumidine. For antigenotoxicity assessment, methyl methanesulfonate (MMS, 44 µg/mL) and hydrogen peroxide (H2O2, 3.5 µg/mL) were added as inducers of chromosome damage. Antioxidant activity was evaluated by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) test. Significantly higher frequencies of micronuclei were observed in cell cultures treated with SC concentrations of 160 and 320 µg/mL in comparison with the negative control, demonstrating a genotoxic effect. There was no significant difference in the frequency of micronuclei between cell cultures treated with a combination of SC and MMS and those treated only with MMS. On the other hand, a significant reduction in the frequency of micronuclei was observed for V79 cells treated with SC or cernumidine plus H2O2 compared to those treated only with H2O2. Furthermore, SC and cernumidine were able to scavenge free radicals in the DPPH assay. Thus, the protective effect of SC and cernumidine against H2O2 can be attributed to antioxidant activity.
A large number of plant species used in folk medicine have been shown to possess carcinogenic, mutagenic or toxic properties. Plants produce a wide variety of active compounds, including those that can cause DNA damage.1) On the other hand, some plant metabolites have been shown to reduce the incidence of DNA damage.2) Therefore, the identification and characterization of these substances are important for the definition of strategies to reduce the risk of cancer in humans. In this respect, tests evaluating the genotoxicity and antigenotoxicity of these plant products are extremely important.3)
Among the plants used in folk medicine, the genus Solanum, which comprises about 1250–1700 species, is the most economically important genus of the family Solanaceae.4) The specie Solanum cernuum VE. is a shrub or small tree found in the southwestern states of Brazil. The plant, popularly known as “panaceia”, has a wide range of applications in traditional medicine, including the treatment of urinary disorders, gonorrhea and skin infections and as antimicrobial and antifungal agent.4–7) Furthermore, activity against gastric ulcer8) and antineoplastic activity of the constituents of the dichloromethane extract have been reported in the literature.9)
The bioactive compounds of S. cernuum (SC) can produce both desirable and undesirable effects.10,11) Toxicity is dependent on factors such as cultivar, growth conditions, light, and type and concentration of the present compounds including guanidine alkaloids.6) The anti-ulcer activity of the hydroalcoholic extract of SC has been attributed to the presence of flavonoids and saponins and among these substances the flavonoids are known for their ability to scavenge oxygen free radicals, which play an important role in the process of ulceration of the gastrointestinal mucosa.8) Lopes et al. isolated from SC, the following compounds: the alkaloid cernumidine; the flavonoid glycosides hyperin, quercitrin and afzelin; and caffeic acid. Cernumidine has been shown to inhibit the production of interleukin-8 by HT-29 colon carcinoma cells, a fact encouraging further research on the prevention and treatment of gastric cancer.12)
In view of the importance of SC as a medicinal plant, it is necessary to evaluate the cytotoxic and genotoxic potential of SC and one of its major component, the alkaloid cernumidine, as well as their possible preventive effect against DNA damage induced by different mutagenic agents.
Leaves of SC were collected in Teresópolis, State of Rio de Janeiro, Brazil in December of 2011. Plant material was identified by Dr. Milton Groppo Jr., Department of Biology, Faculty of Philosophy, Sciences and Letters of Ribeirão Preto—University of São Paulo, SP, Brazil, where a voucher specimen was deposited (SPFR: 15118). Leaves were air dried at 45°C in an oven with circulating air and powdered using a knife mill. One kilogram of plant biomass was submitted to exhaustive maceration using ethanol–water 7 : 3. After filtration, the obtained extract was concentrated under vacuum and lyophilized to furnish 162.8 g (16.3%) of the crude hydroalcoholic extract. Fifty grams of the crude extract were submitted to liquid–liquid partition using solvents of increasing polarity: hexanes (Hex), dichloromethane (DCM), ethyl acetate (EtOAc) and n-butanol (BuOH). All fractions were evaporated to dryness under reduced pressure to yield the following fractions: Hex (1.39 g; 2.78%), DCM (1.84 g, 3.68%), EtOAc (2.89 g, 5.78%), BuOH (10.20 g, 20.40%) and water (27.14 g, 54.28%). EtOAc fraction furnished, by successive open chromatographic columns, isoferulic acid, quercitrin and afzelin. The BuOH fraction showed orange spots in TLC when sprayed with Dragendorff reagent, suggesting the presence of alkaloids. Thus, 1.3 g of BuOH fraction was diluted to 5 mL of methanol (MeOH), loaded on a Sephadex LH-20 (25–100 µm Sigma-Aldrich) column (4 cm i.d.×35 cm) and eluted with MeOH under a flow rate of 3 mL/min, and fractions volume of 7.5 mL. Fractions 18 to 24 were combined after TLC analyses furnishing 572 mg (44%), which was further submitted to purification by preparative liquid chromatography. The LC system (Shimadzu Prominence) consisted of preparative HPLC with two pumps (LC-6 AD), dual wavelength UV detector SPD-20 A, degasser DGU-20 A5, fraction collector FCR-10 A and module CBM-20 A controlled by LC-Solution software. A Phenyl (C-18) column (Shimadzu, 5 µm, 4.6×250 mm) was used as the stationary phase. The mobile phase was composed of water (Phase A) and acetonitrile (Phase B) in a gradient elution: 0–30 min, 10–35% phase B; 30–35 min, 35–90% phase B; 35–40 min, 90–10% phase B, which was kept until 45 min to re-equilibrate the column. The flow rate was set at 9 mL/min and detection at 257 and 324 nm, furnishing 82.94 mg of cernumidine (E)-N-(1-carbamimidoylpyrrolidin-2-yl)isoferulamide. The chemical structures of all the isolated compounds were established by 1H- and 13C-NMR analyses in comparison with published data (Fig. 1).

Phytochemical analysis of the crude extract was carried out by reversed-phase HPLC-UV-diode array detector (DAD) profiling (Fig. 2). HPLC system consisted of a Shimadzu SCL 10Avp liquid chromatography equipped with a Shimadzu SPD-M10Avp photodiode array detector-DAD. The analysis was performed using Synergi Polar RP (C18) column (Phenomenex, 4 µm, 4.6 mm×150 mm). The mobile phase was composed of 0.01% trifluoroacetic acid (TFA) in water and acetonitrile (MeCN) and the gradient of elution was as described in item 2.1 to isolate cernumidine. The UV data were acquired between 190 and 600 nm and chromatograms were simultaneously recorded at 210, 257 and 324 nm. The chromatographic data were processed using Class VP software (version 5.02).

Stationary phase: Synergi Polar RP column (150 mm×4.60 mm, 4 µm). Mobile phase: (A) 0.01% TFA in water and (B) MeCN. Gradient elution: 0–30 min, 10–35% phase B; 30–35 min, 35–90% phase B; 35–40 min, 90–10% phase B, 40–45 min with 10% of phase B. Wavelength: 257 nm. Fow rate: 1 mL/min.
Solutions of 3 µg/mL of the isolated compounds and crude extract were prepared and injected in triplicate into HPLC. The peak areas of the isolated compounds in comparison with their peak areas in the crude extract were used to calculate their concentrations. The profiles were recorded in absorbance at 257 nm.
Cells and Culture ConditionsChinese hamster lung fibroblasts (V79) were kindly provided by Prof. Dr. Ilce Mara Syllos Cólus (State University of Londrina, PR, Brazil). The cells were maintained as monolayers in plastic culture flasks (25 cm3) in Nutrient Mixture F-10 Ham (HAM-F10) (Sigma-Aldrich, St. Louis, MO, U.S.A.) and Dulbecco’s modified Eagle’s medium (DMEM) (Sigma-Aldrich) medium (1 : 1) supplemented with 10% fetal bovine serum (Nutricell), antibiotics [0.01 mg/mL streptomycin (CAS: 3810-74-0) and 0.005 mg/mL penicillin (CAS: 113-98-4); Sigma-Aldrich] and 2.38 mg/mL Hepes (CAS: 7365-45-8; Sigma-Aldrich) at 37°C in a biochemical oxygen demand (BOD) type incubator. The average cell cycle time was 12 h under these conditions and the cell line was used after the 4th passage.
DNA Damage-Inducing AgentsMethyl methanesulfonate (MMS, CAS: 66-27-3; Sigma-Aldrich) was dissolved in phosphate-buffered saline (PBS, pH 7.4) and used at concentrations of 110 µg/mL (colony-forming assay) and 44 µg/mL (micronucleus assay). MMS is a monofunctional alkylating agent that interacts directly with DNA by transferring charged methyl group radicals. Hydrogen peroxide (H2O2, 30 wt % in water, CAS: 7722-84-1; Merck, Darmstadt, Germany) was dissolved in distilled water (concentration of 3.5 µg/mL) at the time of use. H2O2 is a potent genotoxin converted to the hydroxyl radical and then, due to its high reactivity, is able to induce oxidative DNA damage, including DNA-strand breakage and base modification.13)
Colony-Forming AssayThe cell cultures were treated with SC at concentrations ranging from 9.7 to 5000 µg/mL. Negative (no treatment), solvent (dimethyl sulfoxide (DMSO), 0.5 µg/mL; Sigma-Aldrich) and positive (MMS, 110 µg/mL) controls were included. The cultures were treated for 3 h and 300 cells were seeded per culture flask (three flasks per concentration). The experiments were carried out for 10 d. At the end of the growth period, the culture medium was removed and the cells were washed with PBS, fixed in methanol–acetic acid–distilled water (1 : 1 : 8) for 30 min, and stained with Giemsa (1 : 30 in phosphate buffer, pH 7.0) for 30 min.14)
The colonies formed were counted with a magnifying glass and the survival fraction (FS) was calculated for the different treatments using the following formula:
 |
The concentrations of SC employed in the genotoxicity and antigenotoxicity studies were chosen based on the results of the colony-forming assay using cytotoxicity as a selection criterion. Thus, the genotoxicity of SC was evaluated at concentrations of 40, 80, 160 and 320 µg/mL. For antigenotoxicity assessment, the different concentrations of SC (20, 40 and 80 µg/mL) were combined with MMS (44 µg/mL) and H2O2 (3.5 µg/mL). Cernumidine was used at concentrations of 1.0, 2.0 and 4.0 µg/mL, which correspond to 4.7% of the concentrations of 20, 40 and 80 µg SC/mL, respectively. Negative (no treatment), solvent (DMSO, 0.5 µg/mL), and positive (MMS, 44 µg/mL; H2O2, 3.5 µg/mL) controls were included. The protocols were performed in triplicate on three different days to ensure reproducibility. The treatment designs and cell collection and fixation procedures described by Furtado et al. were used.15)
The criterion proposed by Fenech was used for the analysis of micronuclei.16) A total of 3000 binucleated cells were scored per treatment, corresponding to 1000 cells/treatment/repetition. The nuclear division index (NDI) was determined for 1500 cells analyzed per treatment, for a total of 500 cells per repetition. Cells with well-preserved cytoplasm containing 1–4 nuclei were scored. The NDI was calculated according to Eastmond and Tucker using the following formula17):
 |
Additionally, the cytotoxicity index (CI) of SC was calculated as described by Kirsch-Volders et al.18)
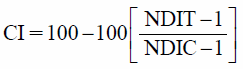 |
The percent reduction in mutagen-induced chromosome damage was calculated according to Waters et al. using the following formula19):
 |
The radical-scavenging activity of SC and cernumidine was determined using DPPH. The principle of this free radical-reducing test is that antioxidants react with the stable free radical DPPH and convert it into 1,1-diphenyl-2-picrylhydrazine. The ability to capture the stable DPPH radical is measured by the decrease in absorbance. The assays were conducted in triplicate in 96-well plates. SC, cernumidine and the positive control (gallic acid, 98.0% purity; Sigma-Aldrich) at concentrations of 1.67 to 66.70 µg/mL in MeOH were individually added to 67.6 mM DPPH in MeOH. The mixture was incubated for 30 min in the dark at 25°C. Remaining DPPH was determined colorimetrically at 517 nm by comparison with methanol (negative control) in a microplate reader. The DPPH free radical-scavenging activity is expressed as the percentage of scavenging activity of mean values obtained in triplicate using the following formula:
 |
The data were analyzed statistically by ANOVA with calculation of statistics and respective p values. In cases in which p<0.05, treatment means were compared by the Tukey’s test and the minimum significant difference was calculated for 0.05.
The quantification of the major compounds in SC by HPLC gave 1.5% of trans-isoferulic acid, 4.7% of the alkaloid cernumidine, 1.3% of quercitrin and 1.6% of afzelin21) (Fig. 2).
Dose-dependent changes in the viability of V79 cells treated with SC were evaluated by the colony-forming assay and the results are shown in Fig. 3. No significant differences were observed between cultures treated with 10, 20, 40 or 80 µg SC/mL and the negative control. In contrast, a significant difference was found for the concentrations of 160, 320, 640, 1280, 2560 and 5000 µg/mL when compared to the negative control, demonstrating a cytotoxic effect.

DMSO, 0.5 µg/mL; MMS, 110 µg/mL. *Significantly different from the negative control group (p<0.05).
The mean frequency of micronuclei and NDI obtained for V79 cells treated with different concentrations of SC are shown in Table 1. Significant differences in the frequency of micronuclei were observed between cultures treated with 160 and 320 µg SC/mL and the negative control, demonstrating a genotoxic effect (Table 1).
| Treatment (µg/mL) | MN frequency | NDI | Reduction (%) | CI |
|---|---|---|---|---|
| Control | 8.66±1.52 | 1.80±0.07 | — | — |
| DMSO | 9.33±3.78 | 1.75±0.04 | — | 6.25 |
| SC 40 | 11.66±2.30 | 1.76±0.04 | — | 5.00 |
| SC 80 | 10.33±2.08 | 1.77±0.04 | — | 3.75 |
| SC 160 | 23.00±4.00a) | 1.76±0,01 | — | 5.00 |
| SC 320 | 26.33±4.16a) | 1.71±0.03 | — | 11.25 |
| MMS | 40.66±3.05a) | 1.74±0.03 | — | 7.50 |
| DMSO+MMS | 36.33±4.04a) | 1.80±0.04 | — | 0.00 |
| SC 20+MMS | 37.66±3.05a) | 1.79±0.05 | — | 1.25 |
| SC 40+MMS | 35.66±3.05a) | 1.78±0.07 | — | 2.50 |
| SC 80+MMS | 35.66±0.57a) | 1.77±0.02 | — | 3.75 |
NDI, nuclear division index; MN, micronucleus; CI, cytotoxicity index; MMS, 44 µg/mL; DMSO, 0.5 µg/mL; SC, hydroalcoholic extract of Solanum cernuum. a) Significantly different from the negative control group (p<0.05).
For antigenotoxicity assessment, the frequencies of micronuclei in cell cultures simultaneously treated with SC (20, 40 and 80 µg/mL) and MMS did not differ significantly from those observed for cultures treated only with MMS. On the other hand, a significant reduction in the frequency of micronuclei was observed in cultures treated with SC and H2O2 when compared to those treated only with H2O2. Additionally, there was a significant reduction in the frequencies of micronuclei in V79 cell cultures treated with cernumidine concentrations of 1, 2 and 4 µg/mL plus H2O2 when compared to the treatment with H2O2 alone. No significant dose-dependent relationship was observed (Tables 1, 2).
| Treatment (µg/mL) | MN frequency | NDI | Reduction (%) | CI |
|---|---|---|---|---|
| Control | 9.00±1.00 | 1.71±0.01 | — | — |
| DMSO | 10.66±1.52 | 1.75±0.04 | — | 0.00 |
| H2O2 | 64.66±6.65a) | 1.72±0.01 | — | 0.00 |
| DMSO+H2O2 | 58.00±4.35a) | 1.74±0.03 | — | 0.00 |
| SC 20+H2O2 | 35.66±4.61a, b) | 1.69±0.03 | 52.10 | 2.81 |
| SC 40+H2O2 | 34.00±1.00a, b) | 1.70±0.01 | 55.08 | 1.40 |
| SC 80+H2O2 | 38.33±4.72a, b) | 1.70±0.00 | 47.30 | 1.40 |
| CER 4.0 | 12.66±0.57 | 1.69±0.04 | — | 2.81 |
| CER 1.0+H2O2 | 33.00±2.00a, b) | 1.72±0.09 | 56.88 | 0.00 |
| CER 2.0+H2O2 | 35.00±2.00a, b) | 1.67±0.02 | 53.28 | 5.63 |
| CER 4.0+H2O2 | 39.33±1.52a, b) | 1.68±0.00 | 45.50 | 4.22 |
NDI, nuclear division index; MN, micronucleus; CI, cytotoxicity index; H2O2, 3.5 µg/mL; DMSO, 0.5 µg/mL; SC, hydroalcoholic extract of Solanum cernuum; CER, cernumidine. a) Significantly different from the negative control group (p<0.05). b) Significantly different from the positive control group (p<0.05).
No significant difference in NDI values was observed between the different treatments and negative control, demonstrating the absence of cytotoxicity under the present experimental conditions (Tables 1, 2).
Figure 4 shows the dose–response curve for SC and cernumidine in the DPPH radical-scavenging assay. SC and cernumidine were evaluated at concentrations ranging from 1.67 to 66.70 µg/mL. SC was able to scavenge free radicals at the highest concentration tested (66.70 µg/mL), with 48.51% scavenging activity. The cernumidine alkaloid was also able to scavenge free radicals at the highest concentration (66.70 µg/mL), corresponding to 33.12% sequestration.

The present results demonstrated the genotoxicity of SC at the two highest concentrations tested. The genotoxicity observed may be due to the presence of cytotoxic alkaloids often found in the genus Solanum. Studies on steroidal glycoalkaloids present in a number of Solanum species have shown significant cytotoxic,22) embryotoxic23) and teratogenic activity.24) According to Kirsch-Volders et al., cytotoxic substances can cause DNA damage depending on their degree of cytotoxicity and in this context,18) the genotoxicity of SC may be attributed to cytotoxicity exerted by alkaloids in higher concentrations. It’s important to emphasize that in according da Silva et al. plants and their isolated compounds can cause beneficial or harmful effects depending on the dose used.25)
Genotoxic effects have also been demonstrated for other Solanum species. Amat et al. reported significant production of chromosome abnormalities induced by S. granuloso-leprosum using the Allium cepa assay.26) A mutagenic effect has also been observed in studies investigating S. palinacanthum DUNAL crude ethanol extract and the glycoalkaloid solamargine by the Ames test.10)
On the other hand, in the present study, SC exerted different effects on genotoxicity induced by the mutagens tested. The extract was able to protect against the DNA damage induced by H2O2, but not by MMS. The alkaloid cernumidine exhibited antigenotoxic activity at all concentrations tested when combined with H2O2. It is important to point out that the alkaloids present in SC belong to the guanidine class.
MMS is a monofunctional alkylating compound that causes direct damage to DNA strands, including the formation of DNA monoadducts as well as crosslinks, causing mutations such as base substitutions. MMS can react with any cellular component with nucleophilic character and the transcriptional responses to this agent suggest the occurrence of damage to RNA and proteins.27) In the present study, SC did not react with or inactivate MMS, which may be due to the inability of the extract to compete with DNA as a target for alkylation caused by this mutagen.
Hydrogen peroxide is a strong oxidizing agent with a great genotoxic potential. This compound can accept two electrons which results in another radical, the hydroxyl (OH) radical, a highly potent oxidant that can indiscriminately oxidize any organic molecule,28) causing damage to macromolecules such as proteins, DNA and lipids. DNA base changes, strand breaks and mutations are generally the consequences when free radicals attack the genetic material.29,30)
Compounds with capacity of scavenging free radicals may also display antigenotoxic property by preventing DNA damage caused by the loss of the redox balance, which leads to oxidative stress. Since both SC and cernumidine exerted antioxidant activity in the DPPH assay, the protective effect of SC against the genotoxicity induced by H2O2 may be attributed in part to the antioxidant/antigenotoxic property of this alkaloid.
Free radical-scavenging ability has also been reported for other Solanum species. Methanol extracts of S. nigrum exerted significant antioxidant activity in different assays, including free radical-scavenging activity in the DPPH test.31) S. paniculatum has also been shown to possess antioxidant capacity, reducing genotoxic damage caused by mitomycin. The antigenotoxic effect was attributed to the antioxidant activity of the extract.2) Munari et al. demonstrated the antigenotoxic activity of S. lycocarpum against damage induced by MMS and attributed this effect to the major glycoalkaloid components, solamargine and solasonine, which reduced DNA damage caused by reactive oxygen species. S. surattense exhibited H2O2-scavenging activity, which was attributed to the presence of phytochemicals such as flavonoids, alkaloids and phenols in the extract.11)
The antioxidant activity of S. surattense may be due to the reduction of hydroperoxides and inactivation of free radicals, or a combination of both.32) The antioxidant activity of S. anguivi was evaluated by the DPPH assay, with this plant exhibiting potent and concentration-dependent free radical-scavenging activity. HPLC analysis revealed the presence of gallic acid, chlorogenic acid, caffeic acid, rutin, and quercetin. The effects observed were thus attributed to the bioactive polyphenolic compounds present in the extract.33)
The observation that SC and cermunidine do not exert a significant dose-dependent protection might be explained by its erratic absorption by the cell membrane and the consequent inconstant bioavailability of the compound into the cell. On the other hand, the assessment of dose–effects is complicated by the fact that many chemoprotective compounds act simultaneously at different levels of protection.34) On this basis, the lack of observation of a dose–response relationship might be attributed to the activation of different mechanisms depending on the natural products dose used.
Thus, the present results showed a genotoxic effect of SC only at the higher concentrations tested (160, 320 µg/mL). Furthermore, this extract exerted no antigenotoxic activity against MMS-induced genotoxicity, but was able to reduce the chromosome damage induced by H2O2. Additionally, one of SC major components, the alkaloid cernumidine, also exerted a protective effect against DNA damage caused by H2O2. Similar free radical-scavenging activity was observed for SC and cernumidine in the DPPH test.
Therefore, the present study agrees with previous studies in which plants of Solanum genus that are used in folk medicine, have shown antioxidant potential and chemoprotective. These results suggest that the antigenotoxic and antioxidant effects of SC may be due, at least in part, to the presence of cernumidine.
Authors are thankful to Dr. Milton Groppo Jr. for plant identification. This work was supported by the São Paulo Research Foundation (FAPESP, Brazil, Grant # 2011/05732-4). Jaqueline Lopes Damasceno was the recipient of a Master’s fellowship from FAPESP (Grant # 2012/07946-4). Mariza de Abreu Miranda also thanks FAPESP for her fellowship (Grant # 2012/00715-7). The authors are also grateful to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for granting fellowships.
The authors declare no conflict of interest.