2016 年 39 巻 6 号 p. 946-952
2016 年 39 巻 6 号 p. 946-952
1.8-Cineole (eucalyptol) is a phytoncide, a volatile organic compound derived from plants. Phytoncides are known to have an anti-inflammatory effect. However, the effects of 1.8-cineole in house dust mite (HDM)-stimulated bronchial epithelial cells are poorly understood. The objective of this study was to assess the effect of 1.8-cineole in HDM-stimulated bronchial epithelial cells and in the HDM-induced murine asthma model. The purpose of the present study is to evaluate the anti-inflammatory effects and mechanism of 1.8-cineole action in HDM-induced airway inflammation. Human bronchial epithelial cells (HBECs) were cultured with Dermatophagoides pteronyssinus (Der p) and 1.8-cineole. Cytokine protein levels, phosphorylation of protein kinases, and intracellular Toll-like receptor 4 (TLR4) expressions were measured. In the murine model, BALB/C mice were sensitized with Der p and were exposed to Der p via intranasal route during the challenge period. 1.8-Cineole was given by inhalation 6 h before the each challenge. Treatment with 1.8-cineole inhibited the Der p-induced cytokine protein expression, phosphorylation of p38 mitogen-activated protein kinase (MAPK) and Akt and intracellular TLR4 expression in HBECs. In the Der p-induced mouse model, airway hyper-responsiveness (AHR) and the number of eosinophils in bronchoalveolar lavage fluid (BALF) was also significantly reduced by 1.8-cineole treatment. The treatment of 1.8-cineole inhibited the increased production of interleukin (IL)-4, IL-13 and IL-17A in BALF after Der p challenge. These results suggest that 1.8-cineole suppresses Der p-induced IL-8, IL-6 and granulocyte macrophage-colony stimulating factor (GM-CSF) production in HBECs. Finally, we confirmed that 1.8-cineole decreases AHR and eosinophilic airway inflammation in Der p-induced asthma mice.
Allergic asthma is a chronic airway inflammatory disease. It is characterized by airway hyper-responsiveness (AHR), pulmonary eosinophilia, antigen-specific T-helper 2 (Th2) cells and mucus hypersecretion.1,2)
House dust mite (HDM) allergy is the most prevalent cause of allergic sensitization that afflicts asthmatics. The human respiratory epithelium is a critical component of the innate immune system, acting as both a physical barrier to the environment and as a modulator of local airway inflammation because of its capacity to synthesize a variety of mediators including cytokines, leukotrienes, nitric oxide, antioxidants and prostanoid.3–5) Several studies have indicated that HDMs induce the release of mediators such as interleukin (IL)-8, IL-6, tumor necrosis factor-α (TNF-α), granulocyte macrophage-colony stimulating factor (GM-CSF), and regulated on activation, normal T cell expressed and secreted (RANTES) from airway epithelial cells.6–9) These mediators trigger the accumulation of inflammatory cells such as eosinophils and neutrophils to perpetuate chronic allergic airway inflammation.
Toll-like receptor 4 (TLR4) ligands are certainly the most studied danger signals in the context of airway allergic inflammation. Recently, it was demonstrated that epithelial cells play a critical role generating allergic inflammation through activation of the TLR4 signaling pathway by HDMs.10)
Several reports show that activating mitogen-activated protein kinase (MAPK), a TLR4-associated signaling pathway, is involved in allergen-induced cytokine expression.11,12) And it was known that exposure to the Dermatophagoides pteronyssinus (Der p) allergen induces rapid activation of phosphatidyl inositol 3-kinase (PI3K), leading to phosphorylation of Akt.13)
1.8-Cineole, the major constituent of eucalyptus oil (eucalyptol), is known chemically as terpenoid oxide. Terpenoid oxide was shown to have anti-inflammatory efficacy equivalent to prednisone in patients with severe asthma in a double blind placebo-controlled trial.14) There has been an increasing interest in the beneficial effects of 1.8-cineole in airway inflammatory diseases. 1.8-Cineole strongly inhibited TNF-α and IL-1β level controlling airway mucus hypersecretion in asthma.15) It also reduced the production of cytokines such as leukotriene B4 (LTB4) and prostaglandin E2 (PGE2) in human monocytes obtained from asthma patients.13) However, the literature contains very little experimental data on how 1.8-cineole affects human epithelial cells stimulated by allergens such as HDMs.
In this report, we examined the effects of 1.8-cineole on Der p-induced upregulation of cytokines expression and activation of p38 MAPK and Akt as its signaling pathway on airway epithelium. We also examined the effects of 1.8-cineole on Der p-induced up-regulation of TLR4 expression in airway epithelium. Finally, we evaluated the effects of 1.8-cineole in Der p-induced asthma mouse model.
1.8-Cineole was purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). The stock solution of 1.8-cineole was suspended in 0.5% dimethyl sulfoxide (DMSO, Sigma) and stored at 4°C. Stock solution was diluted by culture medium (Dulbecco’s modified Eagle’s medium (DMEM)) at final concentration. Der p extract was used from Yonsei University Hospital, (Seoul, Korea).
Cell CultureBEAS-2B cell line, a SV40-transformed human bronchial epithelial cell line, was purchased from the American type culture collection (ATC C) (Manassas, VA, U.S.A.). The cells were grown in DMEM medium with 10% fetal bovine serum and antibiotics (complete culture medium) in a humidified atmosphere at 37°C with 5% CO2. The medium was refreshed every 2–3 d. BEAS-2B cells were plated into 6-well culture dishes (1×106 cells/well).
Cell Viability AnalysisCell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. BEAS-2B cells were treated with 0.1, 1, 10 µg/mL 1.8-cineole for 24 h, and then incubated with MTT (0.5 mg/mL) at 37°C for 4 h. The viable cell number was directly proportional to the production of formazan, which was dissolved in isopropanol and determined by measuring the absorbance at 570 nm using a microplate reader.
Measurement of Cytokine Levels by Enzyme-Linked Immunosorbent Assay (ELISA) and ImmunoblottingAfter exposing BEAS-2B cells to Der p (1–100 µg/mL) and 1.8-cineole (0.1–10 µg/mL) for 24 h, the cell culture supernatants were collected and stored at −70°C until cytokine analysis. Concentrations of IL-6, IL-8 and GM-CSF in cell culture supernatants were determined by ELISA (R&D Systems, Minneapolis, MN, U.S.A.), according to the manufacturer’s manual. And for immunoblotting, phosphorylation of p38 MAPK, p38 MAPK, phosphorylation of Akt and Akt were analyzed by Western blot analysis. After exposing BEAS-2B cells to Der p (100 µg/mL) and 1.8-cineole (10 µg/mL) for 1 h, the cell culture medium was removed, the dishes were immediately rinsed with ice-cold phosphate buffered saline (PBS), and sonicated in radio immunoprecipitation assay (RIPA) buffer prior to protein determination using the Bradford protein assay. Proteins (20 µg/well) from whole-cell lysates were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and blotted onto polyvinylidene difluoride (PVDF) membranes. The membranes were then probed with antibodies for the respective phosphorylated kinases (p-p38 and p-Akt) prior to incubation with horseradish peroxidase (HRP)-conjugated secondary antibodies. The blots were developed using the Super-Signal West Dura chemoluminiscence system (Pierce, Perbio Science, Helsingborg, Sweden). Finally, the membranes were stripped by a 20 min incubation at room temperature with antibody stripping solution (Pierce, Perbio Science), and re-probed with the total amount of the respective kinases (p38, Akt and β-actin). All antibodies were obtained from Cell Signaling Technology (Beverly, MA, U.S.A.). The density of band was measured using Image J software.
Measurement of TLR4 Expression by Flow CytometryAfter exposing the BEAS-2B cells to Der p (100 µg/mL) and 1.8-cineole (10 µg/mL) for 24 h, the cells were washed with stain buffer (BD Biosciences, San Diego, CA, U.S.A.). The cells were permeabilized using fixation/permeabilization concentrate (BD Biosciences) and stained with anti-human TLR4 antibody or anti-mouse immunoglobulin G2a (IgG2a) antibody (eBioscience, San Diego, CA, U.S.A.) as isotype controls. Samples were collected by flow cytometry (Beckman Coulter FC500 MPL, Fullerton, CA, U.S.A.) and the collected data were analyzed using FlowJo 9.6 software (Tree Star, Ashland, OR, U.S.A.).
Development of Allergic Asthma Models and 1.8-Cineole TreatmentSix weeks old female BALB/c mice (18–20 g) were purchased from SLC Inc. (Kotoh-cho, Hamamatsu, Japan). All experiments were performed with the approval of the Institutional Animal Care and Use Committee of the Institute (IACUC) of Laboratory Animal Resources at Seoul National University (SNU-150604-1). Der p was resuspended in PBS and mice were sensitized intranasal injection with 50 µg of Der p in 30 µL of PBS on day 0, 1, 2 and 14. And then mice were challenged by intranasal injection with 50 µg Der p of PBS on day 21, 22 and 23. The stock of 1.8-cineole was suspended in 0.5% DMSO, Sigma. Stock solution of 1.8-cineole was diluted by PBS at 10 mg/mL (1%). Mice inhaled to 10 mg/mL (1%) of 1.8-cineole for 30 min via an ultrasonic nebulizer (Pari, Starnberg, Germany) 6h before each of the Der p challenges. Control mice inhaled PBS instead of 1.8-cineole. The administration was repeated three times during challenge period. This dose of 1.8-cineole was referred to previous study.16) The solution of 1% 1.8-cineole as prepared immediately before use. One day after the last challenge, methacholin bronchial provocation test (MBPT) was evaluated and then mice were sacrificed.
Measurement of Methacholine Hyper-Responsiveness and Analysis of Bronchoalveolar Lavage Fluid and SerumOne day after the last challenge with Der p, airway responsiveness to methacholine was measured using a whole body plethysmograph (OCP 3000, Allmedicus, Gyounggi, Korea), which provides a noninvasive measure of airway responsiveness in mice,17) mice were exposed to increasing doses of methacholine using an ultrasonic nebulizer (Pari, Starnberg, Germany) for 150 s at each concentration. AHR was calculated in enhanced pause (Penh) as previously described.18) The formula used was as follows: Penh=(Te/RT−1)×PEF/PIF, where Te=expiration time (s), RT=relaxation time (s), REF=peak expiratory flow rate (mL/s) and IF=peak inspiratory flow rate (mL/s).
Twenty-four hours after the assessment of airway hyperresponsiveness, mice were sacrificed and bronchoalveolar lavage fluid and lung tissue were obtained. The level of IL-4, IL-13 and IL-17 in BAL fluid was measured using commercially available ELISA Kit (R&D Systems Inc., Minneapolis, MN, U.S.A.) as manufacturer’s guideline. Serum Der p-specific IgG1 was measured by using ELISA.
HistopathologyTo evaluate and compare the severity and character of pathological changes in lung parenchyma, left lungs of mice were fixed in 10% neutral buffered formalin and embedded in paraffin, and 3-mm sections were stained with hematoxylin and eosin (H&E).
Statistical AnalysisResults are expressed as the mean±standard deviation (S.D.), and statistical differences among groups were assessed using Kruskal–Wallis test or Mann–Whitney test. For multiple comparisons Kruskal–Wallis test was used initially. As significant differences were found, Mann–Whitney test was used for the comparison of statistical difference between two groups. Statistical analyses were performed using GraphPad Prizm 5.01 (GraphPad Software, La Jolla, CA, U.S.A.). p-Values <0.05 were considered significant.
Prior to our study, we determined whether Der p (1–100 µg/mL) could enhance IL-8 and IL-6 production in BEAS-2B cells when cells were plated into 6-well culture dishes (2×106 cells/well). The culture supernatants from BEAS-2B cells stimulated with Der p and 1.8-cineole were harvested 24 h after cultivation. Figure 1 shows that Der p (100 µg/mL) induced IL-8 and IL-6 production from BEAS-2B cells when compared with those in control medium (Figs. 1A, B). To determine the concentration of 1.8-cineole, we confirmed that treatment of BEAS-2B cells with 0.1–10 µg/mL 1.8-cineole for 24 h did not cause cell death, as determined by reduction of MTT (data not shown). The levels of IL-8, IL-6 and GM-CSF in Der p (100 µg/mL)-stimulated BEAS-2B cells decreased significantly following treatment with 1.8-cineole (10 µg/mL) (Figs. 1C–E). Therefore, the concentration of 1.8-cineole (10 µg/mL) was chosen based on these results.
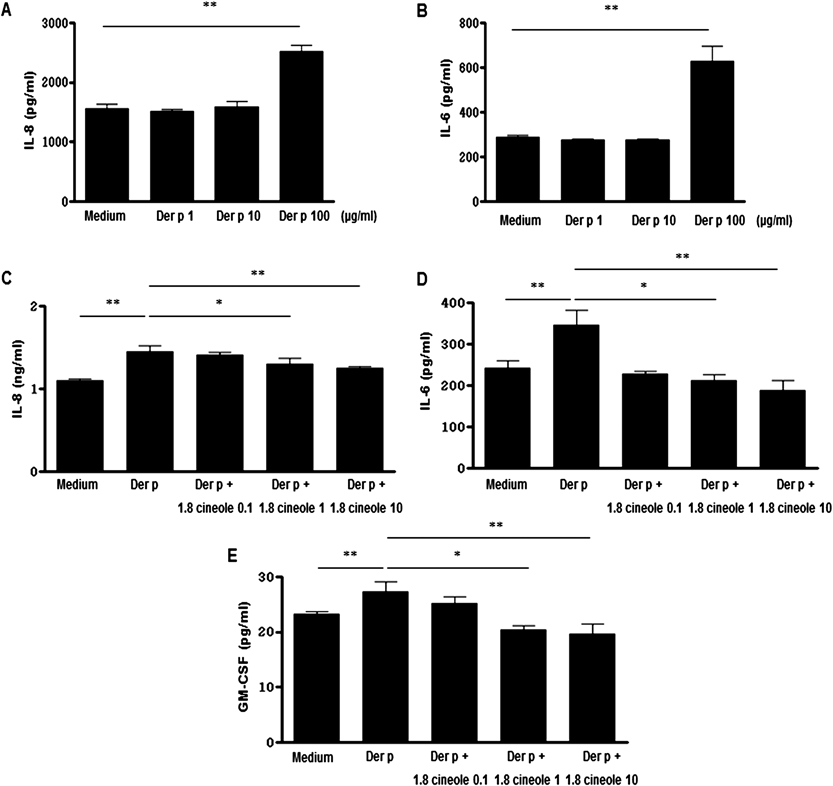
Confluent BEAS-2B cells (2×106 cells/well) were stimulated by Der p (1, 10, 100 µg/mL) and cultured for 24 h. The level of IL-8 (A) and IL-6 (B) in culture supernatant was determined. Confluent BEAS-2B cells (1×106 cells/well) were stimulated by Der p (100 µg/mL) and cultured for 24 h with or without the presence of 1.8-cineole (0.1, 1, 10 µg/mL). The level of IL-8 (C), IL-6 (D) and GM-CSF (E) in culture supernatant was determined. * p<0.05, ** p<0.01.
Many studies have indicated that IL-8, IL-6 and GM-CSF production result mainly from activation of MAPK signaling cascades including extracellular signal-regulated kinase (ERK), c-Jun N terminal kinase (JNK), and p38 MAPK.19) In addition, these productions in BEAS-2B cells also involves activation of Akt.20) Thus, we next examined the effect of Der p on p38 MAPK and Akt activation using a Western blot approach with phosphor specific antibodies that recognize the phosphorylated active forms of these kinases. Phosphorylated p38 MAPK and Akt levels in BEAS-2B cells stimulated with Der p were determined 60 min after stimulation. Both the amount of phosphorylated p38 MAPK and phosphorylated Akt increased in Der p-stimulated BEAS-2B cells compared to medium of BEAS-2B cells (11.47 vs. 34.74%, 10.27 vs. 38.16%). As shown in Fig. 2A, phosphorylated p38 MAPK and Akt in 1.8-cineole plus Der p co-treated BEAS-2B cells were reduced to the level of 1.8-cineole only treated BEAS-2B cells (34.74 vs. 27.18%, 38.16 vs. 25.86%).

Confluent BEAS-2B cells (1×106 cells/well) were stimulated by Der p (100 µg/mL) and cultured for 60 min in with or without the presence of 1.8-cineole (10 µg/mL). Total cellular proteins were extracted from BEAS-2B cells for the detection of phosphorylated p38 MAPK and Akt protein level by Western blot analysis. The density of band was measured using Image J program (A). Experiments were performed in three independent experiments with essentially identical results. And confluent BEAS-2B cells were stimulated by Der p (100 µg/mL) and cultured for 24 h with or without the presence of 1.8-cineole (10 µg/mL). The expression of TLR4 was determined by flow cytometry. Representative histogram shows the expression of intracellular TLR4 (B) and the frequencies of TLR4-producing cells (C). * p<0.05.
Current research suggests that common parameters of airway allergy caused by HDM exposure such as airway inflammation, Th2 cytokine production, and airway hyper-reactivity are strongly attenuated in TLR4 deficient mice compared with those in wild-type mice.21) In addition, TLR4 signaling intermediates include MyD88, IRAK, TRAF6, and MAPK. To gain insight into the mechanism of Der p recognition by pulmonary epithelial cells, we examined TLR4 expression in Der p-stimulated BEAS-2B cells. TLR4 is constitutively expressed in bronchial epithelial cells, and intracellular localization of this receptor has been described.22) We examined intracellular localization of TLR4 in Der p-stimulated BEAS-2B cells by flow cytometry after 24 h. Figures 2B and C show that Der p induced TLR4 expression from BEAS-2B cells when compared with that in control medium. In contrast, TLR4 expression decreased in the presence of 1.8-cineole in Der p-stimulated BEAS-2B cells.
Airway Inflammation, AHR, Th2 Immune Responses Decreased in 1.8-Cineole Treated in Der p-Induced Asthma MiceFinally, we examine the role of 1.8-cineole in Der p-induced asthma model. The numbers of inflammatory cells from BALF, including eosinophils were significantly reduced in 1.8-cineole treated Der p mice compared to Der p mice (Fig. 3A). The recruitment of inflammatory cells into the lungs of mice was also investigated by histopathological studies. In PBS and 1.8-cineole treated mice, few inflammatory cells appeared around respiratory epithelium (Figs. 3Ba, b). Der p mice had significant influx of inflammatory cells including eosinophils and lymphocytes around respiratory epithelium after the challenge (Fig. 3Bc). However, the infiltration of inflammatory cells including eosinophils were found to be reduced significantly in 1.8-cineole treated Der p mice (Fig. 3Bd). We examined AHR with methacholine inhalation. AHR resistances were measured as the Penh (Enhanced Pause) values after methacholine inhalation. After exposure to 50 mg/mL of methacholine, Penh in Der p mice group increased by 183% versus PBS mice group. However, in 1.8-cineole treated Der p mice, Penh was significantly reduced by 63% versus the Der p mice group (Fig. 3C). In serum, Der p-specific IgG1 levels were also significantly reduced in 1.8-cineole treated Der p mice (Fig. 3D). In BALF, the levels of IL-4, IL-13 and IL-17A were found to be significantly reduced by 1.8-cineole treatment in Der p mice (Figs. 3E–G).

The number of inflammatory cells including eosinophils in BALF collected from PBS challenged mice (PBS), PBS challenged mice treated with 1.8-cineole (1.8-cineole 1%), Der p challenged mice (Der p) and Der p challenged mice treated with 1.8-cineole (Der p+1.8-cineole 1%) were counted (A). Lung histology after allergen challenge (a: PBS; b: 1.8-cineole 1%; c: Der p; d: Der p+1.8-cineole 1%, H&E stain, ×400), high numbers of infiltrated inflammatory cells (arrow) in the Der p mice group (B). Mch hyper-responsiveness was measured 24h after the last Der p challenge. Results are presented as the mean±S.D. (C). The levels of serum Der p-specific IgG1 48 h after the last challenge were examined (D). The levels of IL-4 (E), IL-13 (F) and IL-17 (G) 48 h after the last challenge were examined. The levels of cytokine were detected with enzyme-linked immunosorbent assay. * p<0.05, ** p<0.01.
HDMs such as Der p induce expression of inflammatory cytokines and also participate in various signaling pathways. The regulation of HDM-induced cytokine production is a key step in immunity-related diseases. HDMs stimulate IL-8, IL-6 and GM-CSF release from epithelial cells.8) IL-8 is an important activator and chemoattractant for polymorphonuclear leukocytes and has been implicated in a variety of inflammatory diseases such as asthma.23) IL-6 is another key cytokine involved in chronic airway inflammation. An increased level of soluble IL-6 receptor is observed in the airways of patients with allergic asthma compared to that in control individuals.24) IL-6 induces expansion of Th2 cells and suppresses T regulatory cell activity.24,25) GM-CSF released from bronchial epithelial cells of asthmatic patients can prolong the survival of eosinophils and enhance the release of mediators from those cells, contributing to the pathogenesis of airway hypersensitiveness.26,27) HDM-stimulated airway epithelial cells also secrete large amounts of GM-CSF, RANTES and Eotaxin. These pro-inflammatory, pro-Th2 cytokines as well as chemokines recruit and activate Th2 cells, granulocytes (eosinophils, neutrophils, and basophils) and dendritic cells.28)
Several reports have demonstrated that the specific cellular signal transduction pathways that upregulate cytokine production involve signaling via the MAPK such as ERK1/2, p38 MAPK, and JNK to activate the transcription factors nuclear factor-kappaB (NF-κB) and activator protein-1 (AP-1).29) However, recent reports demonstrate that the PI3K/Akt pathway is also involved in allergic asthma both in vitro and in vivo, suggesting that the PI3K/Akt pathway is a potential target for asthma therapy.30) It has also been reported that activating both MAPKs and Akt is important for upregulating IL-6, IL-8 and GM-CSF expression in Der p-stimulated BEAS-2B cells.
The TLR4 ligand is the most studied pattern-recognition receptor in the context of airway allergic inflammation.31) Expression of TLR4 on airway epithelial cells is upregulated after airway challenge with an HDM extract.32) The TLR4 trigger of epithelial cells by the HDM extract promotes secretion of pro-inflammatory cytokines and pro-Th2 cytokines such as thymic stromal lymphopoietin, GM-CSF, IL-25, and IL-33.33) Furthermore, TLR4 knockout mice attenuate HDM-induced eosinophilia, Th2 responses, and airway hyper-responsiveness. In addition, inhaling a TLR4 antagonist to target exposed epithelial cells suppresses the features of asthma, including bronchial hyper-reactivity.34) These findings reflect the pivotal position of epithelial cells in the generation of allergic inflammation through activation of the TLR4 signaling pathway by HDMs.29) TLR4 is constitutively expressed in bronchial epithelial cells, and intracellular localization of this receptor has been described.22) Thus, we detected expression of intracellular TLR4 in a human bronchial epithelial cell line by permeabilization. As a result, we showed that intracellular TLR4 expression increased in Der p-stimulated BEAS-2B cells.
1.8-Cineole may have clinically relevant anti-inflammatory activity in bronchial asthma. IL-1β induces mucus and airway secretions and activates lymphocytes and monocytes/macrophages.35) 1.8-Cineole inhibits IL-1β-stimulated production of TNF-α36) and LPS-stimulated production of IL-1β in monocytes to a similar extent compared to that of budesonide.14) However, the literature contains very little clinical or experimental data on 1.8-cineole in airway epithelial cells.
We explored the possibility that 1.8-cineole affects increased expression of pro-inflammatory cytokines in Der p-stimulated BEAS-2B cells. Our results show that 1.8-cineole inhibited Der p-induced IL-8, IL-6 and GM-CSF production in BEAS-2B cells. We next examined the possibility that 1.8-cineole affects Der p-induced phosphorylation of p38 MAPK and Akt in BEAS-2B cells. We observed an inhibition of increased phosphorylation of p38 MAPK and Akt by 1.8-cineole in Der p-stimulated BEAS-2B cells. However, other molecules involved in the MAPK and Akt signaling pathways should be examined. We also evaluated the possibility that 1.8-cineole affects Der p-induced TLR4 expression. Interestingly, our results showed that 1.8-cineole reduced intracellular expression of Der p-induced TLR4 from BEAS-2B cells.
Finally, we want to measure the effect of 1.8-cineole in Der p-induced asthma mouse model. It was known that rosemary extract at least partially, prevent allergic airway inflammation induced by HDM.37) However, because rosemary extract contains a range of volatile materials and AHR was not present in this study. In addition, in this study, it was shown that rosemary extract is only inhibited the expression of IL-13 enhanced by HDM. Therefore, 1.8-cineole effect in Der p-induced asthma mice is still completely unknown. As a result of our experiment, we demonstrated that airway eosinophilic inflammation, AHR, Th2 and Th17 cytokines decreased in 1.8-cineole treated in Der p induced asthma mice.
Our results demonstrate that 1.8-cineole inhibits Der p-induced cytokines expression including IL-8, IL-6 and GM-CSF in a human bronchial epithelial cell line by suppressing activation of p38 MAPK and Akt. We suggest that these results may have occurred by modulating TLR4 expression in BEAS-2B cells.
In summary, our results support that 1.8-cineole inhibits cytokines production by suppressing activation of p38 MAPK, Akt signaling, and TLR4 expression in Der p-stimulated BEAS-2B cells as a mechanism of animal experiments. Therefore, we think that the therapy using 1.8-cineole as in inhalation vapours helps to control airway inflammation in asthma patients instead of steroid with the high level of negative side effects.
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2009-0072738) and National Project for Personalized Genomic Medicine, Ministry for Health & Welfare, Republic of Korea. (A111218-PG01). Allergen extract was provided from Research Center for Standardization of Allergic Diseases, supported by a Grant from the Korea Healthcare Technology R&D Project, Ministry of Health, Welfare & Family Affairs, Republic of Korea (A092076).
The authors declare no conflict of interest.