2016 年 39 巻 8 号 p. 1338-1346
2016 年 39 巻 8 号 p. 1338-1346
Nanoparticles (NPs) containing cationic monovalent lipids such as 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP) and N-(1-[2,3-dioleyloxy]propyl)-N,N,N-trimethylammonium chloride (DOTMA), have been widely used for the delivery of nucleic acid such as small-interfering RNA and polypeptide to cells as cancer therapies and vaccine development. Several previous reports have suggested that cationic liposomes induce reactive oxygen species (ROS) and ROS-mediated toxicity in cells. Here, we systematically investigated the effects of DOTAP- or DOTMA-containing NPs without any cargo on the human carcinoma cells, HepG2. Treatment with NPs containing DOTAP or DOTMA increased the production of cellular ROS, such as H2O2 and lipid peroxidation, in HepG2 cells and concomitantly decreased cell viability. These effects were dependent on the lipid concentration, surface density of cationic lipids, and particle size of NPs. However, neutral NPs consisting of 1,2-dioleoyl-3-phosphocholine did not elicit the effective ROS generation or cell death regardless of the lipid concentration and particle size. The present study suggests that DOTAP- and DOTMA-NPs are able to induce cancer cell death through production of ROS in the absence of any therapeutic cancer reagents. These results also provide a rational background for the design of delivery systems using cationic lipid-based NP formulations.
Cationic nanoparticles (NPs) are widely used to deliver nucleic acid into targeted cells due to the multiple cationic charges on the NPs interact with polyanionic nucleic acids and form lipoplexes.1) As a cargo of these NPs, small-interfering RNAs (siRNAs) are frequently adopted as an effective strategy for cancer treatment due to their suppression of cancer-related gene (e.g., c-Myc) expression.2) NPs are formulated with cationic lipids, such as monovalent N-(1-[2,3-dioleyloxy]propyl)-N,N,N-trimethylammonium chloride (DOTMA), 1,2-dioleyl-3-trimethylammonium-propane (DOTAP), dioctadecylamidoglycylspermine, and multivalent 3β-(N-[N′,N′-dimethylaminoethane]-carbamoyl)cholesterol, which are used by themselves or by mixing with other lipids and polymers.3) Among these, DOTAP- or DOTMA-containing NPs have been well characterized in studies of delivery systems and preclinical applications.
In addition to nucleic acids, DOTAP has also been used for polypeptide antigen delivery as a cancer vaccine to promote cell-mediated immunity, in which DOTAP plays a role as an adjuvant.4) Similar to siRNA delivery, polypeptide–DOTAP systems are important in the studies of cancer therapies and therefore various formulations containing DOTAP have been developed.4,5) Regarding the molecular mechanisms through with polypeptide-loaded cationic NPs function as cancer vaccines, E7 peptide–DOTAP NPs have been shown to induce the production of reactive oxygen species (ROS) in bone marrow-derived dendritic cells.6) In addition, cationic liposomes containing DOTAP or stearylamine have been suggested to induce ROS and concomitant apoptosis and/or toxicity in cells.7–9) DOTMA also has the capacity for effective siRNA delivery with other cationic polymers (such as polyethylenimine) or receptor-targeted peptides as binary and ternary complexes.10,11) In addition to siRNA, DOTMA-containing particles have been used for gene delivery vectors as lipo(poly)plexes or polyplexes.12) These gene delivery methods using DOTMA-NPs have been shown to effectively stimulate the immune response for carcinoma treatment and are, therefore, promising for clinical applications.13)
ROS are considered so-called “double-edged sword” in cells. Generally, it has been known to be harmful by-products of cellular oxygen metabolism and have been implicated in the pathogenesis of various disorders, such as cancer, diabetes, atherosclerosis, ischemia–perfusion injury, and inflammation including aging.14–16) On the contrary, at relatively low concentrations, ROS acts as a secondary messenger, mediating cellular responses to many physiological stimuli,17) and modulates the innate immune response to virus infection in human epithelium cells.18)
In the present study, we further examined the effects of cationic lipids on ROS production and cytotoxicity in HepG2 human carcinoma cells using DOTAP- and DOTMA-containing NPs without any cargo. Our results could facilitate the rational design of cationic lipid-based NP formulation for various types of delivery systems.
DOTAP, DOTMA, 1,2-dioleoyl-3-phosphocholine (DOPC), 1,2-dioleoyl-3-phosphoethanolamine (DOPE), 12 : 0 lyso-phosphatidylcholine (lyso-PC), and 1,2-dioleoyl-3-phosphoethanolamine-N-(carboxyfluorescein) (18 : 1 PE-CF) were purchased from Avanti Polar Lipids, Inc. (Alabaster, AL, U.S.A.). 2,2,6,6-Tetramethylpiperidin-1-piperidinyloxy (TEMPO), diphenyliodonium chloride (DPI), chlorpromazine hydrochloride, and genistein synthetic were purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.). The Amplex Red assay kit containing horseradish peroxidase and standard H2O2 solution was acquired from Invitrogen (Carlsbad, CA, U.S.A.). Protease inhibitor complex and trypsin–ethylenediaminetetraacetic acid (EDTA) solution were purchased from Roche Diagnostics (Mannheim, Germany). The human hepatocellular carcinoma cell line HepG2 was obtained from the Korean Cell Line Bank (Seoul, Korea). HepG2 cells were maintained in RPMI-1640 medium (Invitrogen) supplemented with 10% fetal bovine serum (FBS).
Preparation of NPsCationic lipid-containing liposomes were prepared using freezing-thawing followed by the extrusion method as previously described.19) Briefly, lipid films were made in a glass tube by evaporation of chloroform under a stream of nitrogen gas. Dry lipids were hydrated with distilled water by brief sonication in a bath sonicator. To obtain homogeneous liposomes, the dispersion was frozen and thawed five times and extruded through two polycarbonate membranes with pore sizes of 100, 200, or 400 nm (Hamilton Co., Reno, NV, U.S.A.). To obtain cationic lipid-containing mixed micelles, 50 mol% DOTAP (or DOTMA) and 50 mol% 12 : 0 lyso-PC were used as lipid components, as previously described,20) and were suspended in water. To prepare fluorescent NPs, 0.5 mol% 18 : 1 PE-CF was incorporated into NPs during the formulation. The particle sizes and zeta (ζ) potentials of liposomes and micelles were measured using a Zetasizer Nano ZS (Malvern Instruments Inc., Westborough, MA, U.S.A.). All cationic NPs including DOTAP/DOPC-liposomes had zeta potentials of 40–45 mV except for DOTAP/DOPE-liposomes, which had approximately 29–36 mV, depending on the concentrations of DOPE. The particle sizes of liposomes were observed to be marginally different from each pore size of the membrane. The particle sizes of mixed micelles were determined to be approximately in the range of 20–30 nm.
Cell Culture and Treatment of NPsHepG2 cells were seeded into six-well plates approximately at a density of 4.0×105 cells/well in culture medium, and then cultured for 1 d at 37°C. After that, the cells were incubated with the formulated NPs for each indicated period in the RPMI-1640 medium without FBS to prevent a decrease in the zeta potential of NPs and/or disintegration of NPs.21) At each predetermined time, the cells were centrifuged (200×g for 5 min) and were washed twice with phosphate-buffered saline (PBS) to remove the medium and NPs. The cells were then detached using 1×trypsin–EDTA for 1 min, and the washing step was repeated twice using 10 mL PBS after termination of trypsin reaction by the culture medium solution. All treatments with the NPs were performed in a humidified incubator at 5% CO2.
Fractionation of CellsCytosolic and microsomal fractions were obtained as previously described.22) Briefly, the cultured cells were re-suspended in a buffer solution (250 mM sucrose, 20 mM N-(2-hydroxyethyl)piperazine-N′-2-ethanesulfonic acid (HEPES), pH 7.4, 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, and 1× protease inhibitor complex) on ice for 20 min after harvest. The cells were then homogenized, and the lysates were centrifuged at 750×g at 4°C for 10 min to remove non-lysed cells and nuclei. The supernatant was then centrifuged at 100000×g at 4°C for 1 h. The resulting supernatant and pellet were saved as the cytosolic and membrane fractions, respectively.
Measurement of ROSThe levels of intracellular hydrogen peroxide (H2O2) and lipid peroxidation (LPO) in the membrane fraction were used as criteria for ROS. After cell fractionation, the amount of H2O2 generated in the cytosolic fraction was measured spectrofluorimetrically using an Amplex Red (AR) assay kit according to the manufacturer’s instructions. Fluorescence was recorded by AR assays at an emission wavelength of 585 nm and an excitation wavelength of 571 nm. LPO in the membrane fraction was estimated by measuring thiobarbituric acid-reactive substances using malondialdehyde as a standard, as described previously.23)
Other MethodsThe concentrations of DOTAP and DOTMA were measured by weighing the dry lipids. DOPC and lyso-PC concentrations were determined by phosphorus assay.24) Cell viability was assayed using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay kit (Sigma-Aldrich) according to the manufacturer’s instructions. The fluorescence of cells after treatment with 18 : 1 PE-CF-containing NPs was observed using Motic BA410 (400×) fluorescence microscope (Hong Kong). Statistical data are expressed as the mean±standard deviations of at least three independent trials. Two-tailed Student’s t-tests were used for statistical analysis. A p-value <0.05 was considered significant.
Previous studies have shown that cationic liposomes induce ROS and concomitant apoptosis and/or toxicity in cells.7–9) We first verified the suggestions using DOTAP- or DOTMA-containing liposomes without any cargo. When ROS generation was measured in a time-dependent manner, the increase in H2O2 and LPO levels reached a plateau at approximately 40–60 min after treatment of DOTAP-liposomes (Fig. 1A). DOTMA-liposomes also showed time-dependent results similar to those of DOTAP-liposomes (results not shown). However, no changes were observed after treatment of liposomes with 100% DOPC, a neutral phospholipid, as a control.
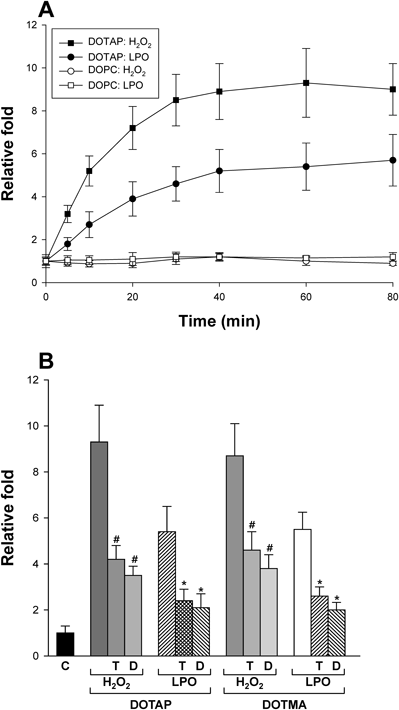
(A) The relative fold change in H2O2 (■, □) and LPO (●, ○) levels were analyzed with cytosolic and membrane fractions of cells in a time-dependent manner after treatment with DOTAP-liposomes for each indicated time. The diameter of liposomes was 100 nm. The concentration of DOTAP- or DOPC-liposome was 100 µM. The y axis represents relative fold, and the ROS level in untreated cells was set to one (1) as a control. (B) ROS levels were measured after treatment with each indicated liposome (with a diameter of 100 nm) for 60 min and were depicted relatively. HepG2 cells were pre-incubated with TEMPO or DPI for 20 min and then treated with liposomes. The treated concentrations of DOTAP and DOTMA were 100 and 200 µM, respectively. C, T, and D represent control, TEMPO, and DPI, respectively. #,* p<0.05, compared with cationic liposome-treated cells in the absence of TEMPO and DPI.
To confirm ROS generation by cationic lipids in HepG2 cells, the experiment was repeated in the presence or absence of anti-oxidative reagents. As shown in Fig. 1B, treatment with 100 µM DOTAP-liposomes (diameter of 100 nm) increased H2O2 production and LPO in the cells by approximately 9- and 5-fold, respectively. DOTMA-liposomes also stimulated ROS generation with similar degrees to those of DOTAP-liposomes although 200 µM DOTMA-liposomes were treated. In contrast, when 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) and diphenyleneiodonium chloride (DPI) were pre-incubated with the cells as a free-radical scavenger and antioxidant, respectively, significant inhibition of cationic NP-induced ROS production was observed for both DOTAP and DOTMA, demonstrating the radical-mediated production of LPO and even H2O2, as previously suggested.25) These results were similar to those using dendritic cells and DOTAP/E7 NPs, as described above. Therefore, taken together, our results suggest that ROS is generated in HepG2 by the specific interactions of cationic NPs with the cells.
Lipid Concentration-Dependent ROS GenerationTo obtain more insight into the effects of cationic lipids on the cells, ROS was measured as a function of lipid concentrations in cationic liposomes with a diameter of 100 nm. Figure 2 shows that ROS levels increased as the lipid concentration increased, reaching a maximum steady-state level at around 100–200 µM of DOTAP-liposomes. For DOTMA-liposomes, a rather higher concentration was required to generate the maximum level of ROS. However, the levels of ROS produced at the higher concentration of DOTMA-liposomes were similar to those of DOTAP-liposomes. Because both cationic lipids are monovalent, it is not clear why there was such a difference in efficacy between DOTAP- and DOTMA-liposomes. As a control experiment, no noticeable ROS was produced by treatment with DOPC-liposomes although concentrations above 400 µM were not tested. These results confirmed the specific cellular effects of cationic lipids.
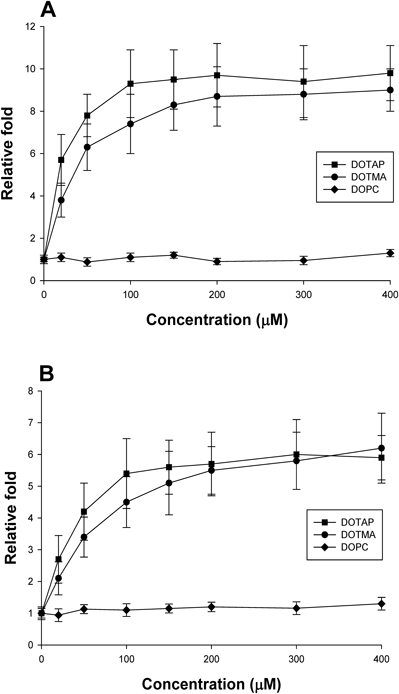
H2O2 (A) or LPO (B) levels were measured in HepG2 cells after treatment with each of the indicated concentrations of liposomes (with a diameter of 100 nm) for 60 min and were plotted with a relative scale. The ROS level in untreated cells was set to one (1) in y axis. The x axis represents the total lipid concentration of liposomes used to treat cells.
Based on the results of the specific interactions between the cationic lipids and the cells, as suggested above, we measured H2O2 generation as the surface positive charge of DOTAP-liposomes decreased by replacing DOTAP with a neutral lipid, DOPC or DOPE. As shown in Fig. 3A, H2O2 and LPO levels decreased with decreasing the positive charge of NPs by addition of neutral lipids up to 50 mol% at the expense of DOTAP; DOPE caused more significant reduction than DOPC. This result confirmed the findings presented in Fig. 1 and was reasonable considering that the DOPC-liposomes had no effect on ROS generation in HepG2 cells. As a control experiment, all liposomes used showed marginal differences in their particle sizes, and formulations with more than 50 mol% DOPE were not used due to changes in the particle size of liposomes (results not shown). However, when the experiment was repeated even under the same cationic lipid concentrations, the DOTAP/DOPC (or DOTAP/DOPE, 1 : 1 by molar ratio)-liposomes produced less amount of H2O2 than DOTAP-liposomes (Fig. 3B). Therefore, these results suggest that the surface density of positive charge on the liposomes is critical for ROS generation in the cells.
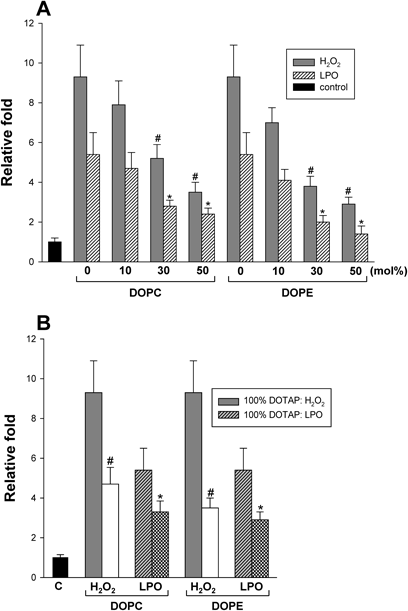
(A) The amounts of ROS were measured with increasing concentrations of DOPC or DOPE of liposomes up to 50 mol% at the expense of DOTAP. #,* p<0.05, compared with 100% DOTAP-liposome-treated cells. (B) ROS levels produced in HepG2 cells were compared between DOTAP/DOPC (and DOTAP/DOPE)-liposomes and DOTAP-liposomes under the same cationic lipid concentration (100 µM as a DOTAP concentration). The concentration of DOPC or DOPE in liposomes was 50 mol%. #,* p<0.05, compared with 100% DOTAP-liposome-treated cells. C represents control. All liposomes had a diameter of 100 nm.
It has been known that the NPs enter the cells via endocytosis, pinocytosis, or phagocytosis pathways, and thus the NPs are often accumulated in endosomal compartments.26) Therefore, the size of NPs may be important to the cellular uptake efficiency and/or the possible role(s) of these NPs, such as generation of ROS. To test this probability, ROS levels were measured by varying the particle size. As shown in Fig. 4A, ROS production was reduced as the size of DOTAP-liposomes increased. Interestingly, mixed micelles containing DOTAP (or DOTMA) and lyso-PC (1 : 1, by molar ratio) increased the levels of H2O2 and LPO by a maximum of approximately 20- and 14-fold, respectively, at a total lipid concentration of 100 µM. These results do not seem to be consistent with those of Fig. 3 because the mixed micelles stimulated ROS generation despite the reduction in surface cation density following incorporation of neutral lyso-PC. Moreover, the mixed micelle-induced production of ROS seemed to reach a maximum level at a lower lipid concentration than that of DOTAP (or DOTMA)-liposomes (Fig. 4B). However, the mixed micelles consisting of 50% DOPC and 50% lyso-PC had no effect. Therefore, taken together, these results suggest that the particle size of cationic NPs plays an important role in ROS production, similar to the effects of the lipid concentration and the surface cationic density on NPs. However, this direct comparison between liposomes and mixed micelles may not be reasonable due to their different particle sizes and different particle concentrations, even at the same cationic lipid concentration. Based on these results, we expect that if micelles were composed of 100 mol% cationic lipids, such as stearylamine or a combination of DOTAP and stearylamine, they may produce even more ROS than that generated by the cationic mixed micelles used in this study. As a control experiment to test neutral micelles, micelles composed of 100 mol% lyso-PC were analyzed; these micelles had no effect on ROS production.
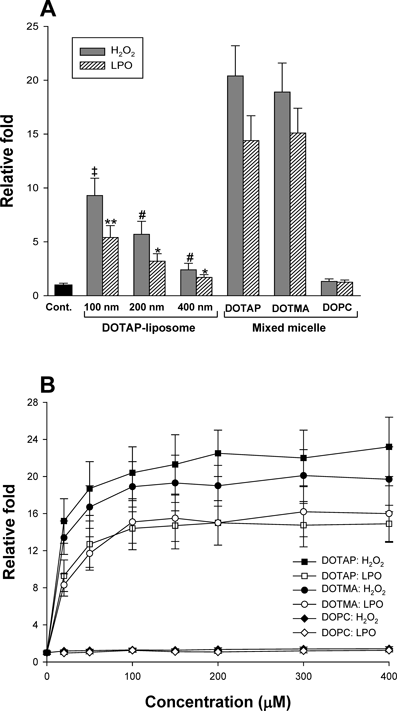
(A) ROS levels were analyzed with increasing diameters of DOTAP-liposomes and with mixed micelles (DOTAP : lyso-PC or DOTMA : lyso-PC, 1 : 1 by molar ratio) at a concentration of 100 µM. #,*: p<0.05, compared with DOTAP-liposomes with a diameter of 100 nm. ‡,** p<0.05, compared with DOTAP (or DOTMA)-micelles. (B) H2O2 (closed symbols) and LPO (open symbols) levels were measured with increasing concentrations of mixed micelles after treatment with micelles for 60 min. The ROS level in untreated cells was set to one (1) on y axis. The x axis represents the total lipid concentration of liposomes used to treat cells.
To examine the correlation between NP-induced ROS generation and subsequently cell death, cell viability was measured in a particle size- and lipid concentration-dependent manner. As shown in Fig. 5A, cell death was stimulated by increasing lipid concentrations of DOTAP (or DOTMA)-liposomes and DOTAP (or DOTMA)/lyso-PC micelles. However, the results also indicated that cell death was not promoted at relatively low concentrations of the cationic-liposomes, despite generation of significant amounts of ROS. Therefore, a certain level of ROS may be required to induce cell death. Here again, the cationic micelles were more effective in the induction of cell death than cationic liposomes, and DOTAP-liposomes with a diameter of 400 nm showed the lowest efficiency of cell death induction. However, DOPC-micelles had almost no effect, even at high lipid concentrations. Therefore, these results imply that the cationic lipid concentration and particle size of NPs play important roles in the cell death of HepG2. In addition, these results suggest that NP-induced ROS is related with cell death, although the cell death was not significant at low concentrations of cationic liposomes. However, it is also possible that other cellular effects of cationic NPs, in addition to ROS, are involved in the induction of cell death. Similar to ROS generation, the relationship between surface charge density on liposomes and cell death was also tested. When DOPC was incorporated into the liposomes at the expense of DOTAP or DOTMA, cell viability increased and cell death was almost not observed in the presence of 50 mol% DOPC (Fig. 5B).
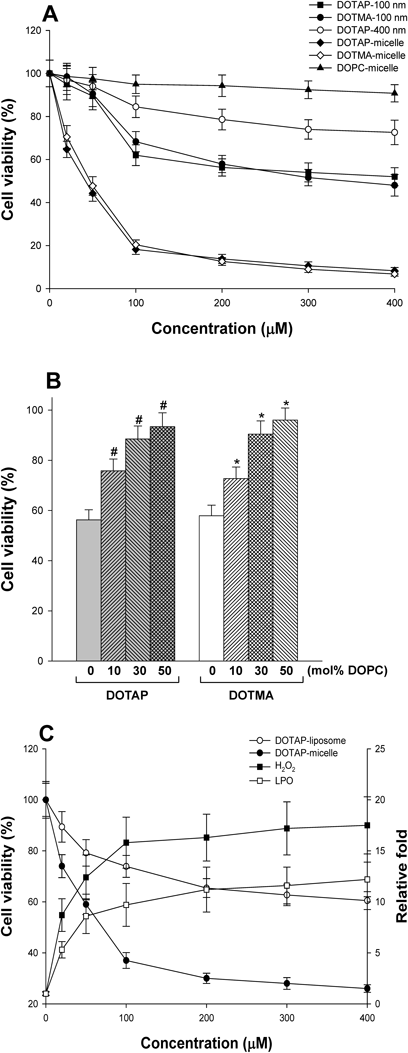
(A) Cell viability was measured with increasing concentrations of NPs using MTT assay. HepG2 cells were treated with each of the indicated concentrations of NPs for 5 h. The diameter of DOTAP-liposomes was 100 or 400 nm. The viability of untreated cells was set to 100%. All mixed micelles were composed of each cationic lipid (50 mol%) and lyso-PC (50 mol%). (B) Cell viability was measured by replacing DOTAP or DOTMA with DOPC in liposomes (a diameter of 100 nm). The total lipid concentration was 200 µM in all liposomes. The numbers represent mol% DOPC. #,* p<0.05, compared with 100% DOTAP (or DOTMA)-liposomes. (C) Cell viability (●, ○) and ROS generation (■, □) in HeLa cells were analyzed with increasing concentration of DOTAP-micelles or DOTAP-liposomes (a diameter of 100 nm). The experimental conditions were the same as those for HepG2 cells.
To test the effects of cationic micelles on carcinoma cells in general, we also examined NP-induced death of HeLa cells, a cervical cancer cell line, and obtained the results similar to those in HepG2 cells. Namely, DOTAP-micelles were more effective at ROS production and subsequent cell death toward HeLa cells than DOTAP-liposomes were (Fig. 5C).
Uptake of NPs into CellsNPs may induce ROS production by interaction with cells on the cell surface and/or within cells. To investigate the possible uptake of NPs into cells, carboxyfluorescein-labeled lipids were incorporated into NPs during the formulation. Figure 6A shows that HepG2 cells emitted fluorescence following treatment with DOTAP-liposomes and DOTAP (or DOTMA)-mixed micelles. Additionally, fluorescence was observed throughout the cells. In contrast, when the cells were treated with DOPC-liposomes, DOTAP-liposomes having a diameter of 400 nm, and DOTAP-micelles after pre-treatment with chlorpromazine, a clathrin-mediated endocytosis inhibitor, the emitted fluorescence were markedly reduced. These results may indicate that NPs were taken up into cells through a certain type of endocytosis that was dependent on cationic lipids and particle size. However, a direct quantitative comparison in fluorescence intensity between different DOTAP-liposomes and the cationic-micelles was not possible under microscopic examination. Also, we could not exclude the possibility that CF-containing NPs were simply attached to cell surface, resulting in observation of fluorescence. Therefore, these results may not provide a direct evidence for the cellular uptake of NPs and the localization of NPs within cells should be confirmed using other dye-staining methods, such as 4′,6-diamidino-2-phenylindole for nuclear staining.
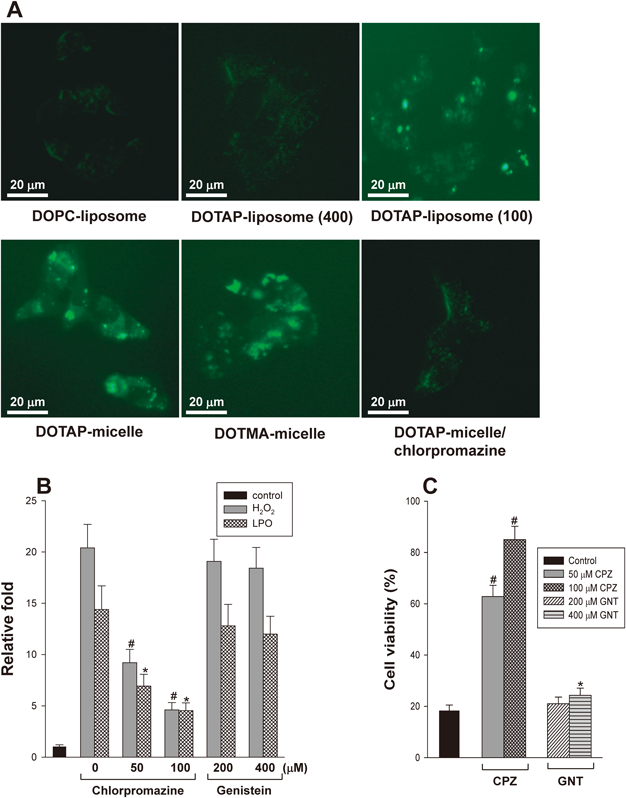
(A) The cellular uptake of NPs into cells was imaged using 18 : 1 PE-CF incorporated into NPs under a fluorescence microscope. The numbers in parentheses represent the diameter (nm) of DOTAP-liposomes. (B) ROS generation by DOTAP-micelles was analyzed in the presence of chlorpromazine and genistein. HepG2 cells were pre-treated with an endocytosis inhibitor for 30 min, and H2O2 and LPO levels were then measured after treatment with DOTAP-micelles for 60 min. (C) The decrease in cell viability induced by DOTAP-micelles was determined in the presence of chlorpromazine and genistein as described in (B). CPZ and GNT represent chlorpromazine and genistein, respectively. #,* p<0.05, compared with DOTAP-micelles in the absence of endocytosis inhibitors as a control. In (B) and (C), the lipid concentration of DOTAP-micelles was 100 µM.
To further demonstrate the uptake of NPs into cells, ROS production and cell viability were measured in the presence of chlorpromazine or genistein, a caveolae-mediated endocytosis inhibitor. The results showed that H2O2 and LPO levels were significantly reduced (Fig. 6B) and that cell death induced by DOTAP-micelles was inhibited (Fig. 6C) following pre-treatment with chlorpromazine. However, genistein showed marginal effects on both assays. These results indicate that DOTAP-micelles were internalized into HepG2 cells by clathrin-mediated endocytosis but not caveolae-dependent way. The results are also similar to the previous report showing that uptake of DOTAP/DNA lipoplexes into A549 pneumocytes and HeLa cells was inhibited by chlorpromazine but unaffected by genistein.27) However, it is unclear how the amount of NP uptake is quantitatively related to the level of ROS generation and the occurrence of cell death in HepG2 cells.
In this study, we systematically investigated the functional roles of cationic NPs in HepG2 cells and concluded that particle size, NP concentration and surface density of the cationic lipid on NPs were important factors in mediating ROS production and cell death in HepG2 cells. In relation to the present results, other reports have shown that cationic liposomes induce ROS-mediated apoptosis in macrophages or WEHI231 cells.7) The formulation of NPs with smaller diameter was also shown to be crucial for improving the efficiency of NP-mediated DNA transfection, according to experiments with poly(lactic acid)- and poly(D,L-lactide-co-glycolide)-NPs.28) Moreover, many studies have revealed that the plasma membranes of cells would select the endocytosis pathway of NPs depending on theirs size, shape, and surface chemistry.29) Nonetheless, in this study, we demonstrated for the first time that mixed micelles containing either DOTAP or DOTMA effectively interact with human hepatocellular carcinoma cells, resulting in cell death through ROS production without any cargo. In addition, the treatment of HeLa cells, a cervical cancer cell line, with the NPs also showed the results similar to those in HepG2 cells. Therefore, the effects of cationic micelles may be generalized to carcinoma cells. Therefore, based on these results, we hypothesize that cationic NPs may exert similar effects in other cancer cell lines and even in normal cells.
One possible mechanism for cationic NP-induced ROS production is through the reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, as previously suggested.30) NADPH oxidase is plasma membrane-bound enzyme complex and it is found in phagosomes of neutrophil as well.30) Mitochondria are another major intracellular source of ROS and it is well-established that the organelle initiates ROS-mediated cell death. Several mechanisms explaining ROS generation have been suggested in mitochondria, which are associated with mitochondrial membrane potential, nitric oxide, and Ca2+ influx.31,32) Recently, Sun et al. suggested that NADPH oxidase activation is an initiator of mitochondrial damage, which eventually results in the death of HepG2 cells.33) Based on these results, we also measured the enzyme activity after treatment with cationic NPs used in this study. However, DOTAP-NPs had marginal effects on the enzyme activation, regardless of particle size or lipid concentration (results not shown). Therefore, NADPH oxidase may not have acted as a trigger for cationic NP-induced ROS generation in this study.
As described above, NPs enter the cells via endocytosis, pinocytosis, or phagocytosis pathways, and then, they are often accumulated in endosomal compartments.26) Subsequently, these NPs either escape endosomes or they are degraded in lysosomes. Although the exact mechanisms remain unknown, ROS may be generated in these pathways on the cytoplasmic or endosomal membranes, especially in the case of cationic NPs.6) Moreover, NPs exit the cell via exocytosis.29) It is likely that the fate of NPs is strictly dependent on their physico-chemical properties such as the size, shape, composition, chemical bond, and surface charge. Regarding the uptake of micelles specifically into cells, Zhang et al. suggested that dynamin- and caveolin-dependent endocytosis is involved in the cellular uptake of polymeric micelles composed of poly(ethylene glycol)–poly(lactic acid) and that these micelles are colocalized with lysosome and microtubulin.34) In addition, Cui et al. reported that doxorubicin-loaded micelles containing monomethoxy–poly(ethylene glycol) are internalized into HeLa cells, release the cargo into the cytoplasm, and enter the nuclei.35) Therefore, we propose that the pathway of micelle uptake may be also dependent on the composition, particle sizes, and/or other physico-chemical properties of micelles. In practice, many experimental evidence have been proposed showing that the uptake of NPs is affected by those properties of NPs.29) However, it is still unclear whether the DOTAP-NPs used in this study were internalized and where the NPs were localized in cells although the result presented in Fig. 6 support the notion that NPs were taken up into HepG2 cells. On the basis of the experiments on replacement of DOTAP with DOPC or DOPE, we believe that surface density of positive charges on the liposomes is crucial for ROS production in the cells: the decrease in the positive charge on NPs by incorporation of the neutral lipids reduced the ROS production (Fig. 3). In addition, the results in Fig. 6 show that DOPC-liposomes were not internalized by cells effectively. Therefore, taken together, these data further suggest that the surface charge density of NPs may be closely related to the cellular uptake of NPs.
In most reports investigating the effects of cationic NPs on cells, including this study, the correlation between ROS levels and cell viability have been analyzed, and their relationships have been successfully revealed. However, ROS production by DOTAP-liposomes did not directly elicit cell death, whereas that by DOTAP-micelles did (Fig. 5). DOTMA-NPs also showed the same relationship. These results suggest that a certain minimum levels of H2O2 and LPO are required to induce the cell death and/or that other types of ROS, which were not tested in this study, are directly related to the decrease in cell viability. It is also unclear whether these phenomena were dependent on the type of cationic lipid and the particle size of NPs, although stearylamine-NPs have shown to induce ROS-mediated apoptosis in cells.7) However, it doesn’t seem that any kind of micelles plays a role in stimulating ROS generation directly after uptake into cells, based on the suggestion that some specified micelles could be used to increase in cell viability under hypoxia.36) Therefore, further analyses using various cationic lipids, including evaluation of NP-induced signaling in cells, should be performed to design effective NP formulations.
In order to achieve various goals in research and therapeutic fields using NP-mediated delivery systems, NPs often contain conjugated reagents, such as antibodies or peptides as well as loaded cargos to elicit efficient targeting to specific cells and disease sites.37,38) These elaborate strategies are needed for enhanced efficacy of material delivery and therefore for reduced side effects in normal cells.39) However, the NPs used in this study did not contain such probes for cellular targeting, although the cationic micelles actually induced death in HepG2 and HeLa cells. Moreover, it is unclear whether the current formulations of NPs may be applicable to animal models of cancer. Therefore, it is not likely that the present formulations of cationic NP can be utilized for any specific applications.
Our current system still has a lot of issues that should be solved. With respect to the treatment duration of cells with NPs, it is unclear why ROS production under the influence of DOTAP-liposomes reached the plateau at 40–60 min (Fig. 1A). This is a kinetically important problem because this time may be related to functional interaction of NPs with cells including the mechanisms of ROS production. Although our data do not provide empirical evidence, a certain time range may be required for cellular attachment and/or uptake of NPs, accumulation in subcellular compartment(s), and ROS production. A difference in lipid concentration-dependent ROS generation between DOTAP- and DOTMA-liposomes (Fig. 2) is also a challenging problem because both cationic lipids have a positive charge on their head group and a similar molecular structure. Although the produced ROS levels were similar for both cationic lipids at their high concentrations (for example, 400 µM), substantial differences in the levels were observed at lower concentrations of lipids. Ether linkages between trimethyl ammonium headgroup and the alkyl chains (as in DOTMA) are thought to result in greater transfection efficiency in comparison with cationic lipids with ester linkages such as DOTAP at identical locations.40) Moreover, it has been proposed that the ether linkages are chemically stable but not biodegradable, whereas the ester linkers are biodegradable by endogenous esterases and are less toxic to cells, but not chemically stable.41) Therefore, these differences in chemical properties may explain the different interactions between the NPs and cells although the precise cellular mechanisms of cationic lipids should be studied further.
NP-induced cytotoxicity, which may be closely related to cationic lipids and sizes of NPs among other factors, is another problem to be considered in the current research. This is because the development of cationic NPs has often been limited by the prerequisite of low cytotoxicity during polynucleotide delivery into cells. Although the cationic charge appears to improve the efficacy of NPs for gene and drug delivery into cells, cationic NPs show well-pronounced cytotoxicity such as disruption of plasma membrane integrity and damage to intracellular organelles including mitochondria, lysosomes, and the nucleus.42) The NP-induced toxic effects on cells are dependent on many characteristics of NPs such as the material, size, shape, composition, surface charge, and surface hydrophobicity, and are often related to extracellular and intracellular production of ROS.42) The efficiency of cellular uptake of NPs also affects the toxicity. Taken together, these observations indicate that the present NPs are cytotoxic via ROS production regardless of the cell type. However, cationic NP-generated ROS can induce apoptosis, thus compromising anti-tumor activity. Therefore, certain strategies to discriminate between cancer and normal cells should be adopted when our system is used in some fields for cancer therapy as described above.
Despite the many weakness and problems with the cationic NPs tested in this study, our data provide a rational background for development of delivery system and facilitate the design of cationic lipid-based NP formulation for cancer therapy, vaccine development, and other applications. In conclusion, our study suggested that cationic lipid-containing NPs induced ROS generation and death in HepG2 cells depending on the lipid concentration, surface density of cationic lipids, and particle size. DOTAP (or DOTMA)-micelles showed the most effectiveness in these assays.
We thank Dr. Leaf Huang (University of North Carolina at Chapel Hill, NC, U.S.A.) for his substantial contributions to conception and design of this study. This research was supported by the Next-Generation BioGreen 21 program (SSAC Grant# PJ011058), Rural Development Administration, Republic of Korea (C.-H. Yun) and by Chonnam National University, 2016–0225 (C.-S. Bae).
The authors declare no conflict of interest.