2016 年 39 巻 8 号 p. 1353-1358
2016 年 39 巻 8 号 p. 1353-1358
Methylmercury (MeHg) is one of the most toxic environmental pollutants and presents a serious hazard to health worldwide. Although the adverse effects of MeHg, including neurotoxicity, have been studied, its effects on immune function, in particular the immune response, remain unclear. This study examined the effects of low-dose MeHg on immune responses in mice. Mice were orally immunized with ovalbumin (OVA) or subcutaneously injected with mite extract to induce a T-helper 2 (Th2) allergic response. They were then exposed to MeHg (0, 0.02, 1.0, or 5.0 mg·kg−1·d−1). Immunization with oral OVA or subcutaneous mite extract increased serum levels of OVA-specific immunoglobulin (Ig) E (OVA-IgE), OVA-IgG1, interleukin (IL)-4, and IL-13, and total IgE, total IgG, and IL-13 when compared with levels in non-immunized mice. However, no interferon (IFN)-γ was detected. By contrast, serum levels of OVA-IgE, OVA-IgG1, IL-4, and IL-13, or total IgE, total IgG, and IL-13 in Th2 allergy model mice subsequently treated with MeHg were no higher than those in MeHg-untreated mice. These results suggest that MeHg exposure has no adverse effects on Th2 immune responses in antigen-immunized mice.
Mercury is widely distributed across the planet and is one of the most serious environmental pollutants. Mercury released into the environment by natural events and various anthropogenic activities, such as coal burning, industrial use, and gold-mining, is an increasing public health concern.1) Metallic and ionic forms of mercury can accumulate in sediments, where they are readily converted to highly toxic methylmercury (MeHg) by microorganisms.2) Bioaccumulation of MeHg occurs within the food chain, and the compound can be found in higher levels in some fish species, including tuna, swordfish, and bonito.3) Clinical studies show that MeHg is the principal mercurial compound that accumulates in fish and is biomagnified in their consumers, thereby exerting toxic effects in various organs and systems.1,4)
Severe neurological disorders occur in victims of MeHg poisoning, which is a known cause of Minamata disease.5) Other than the nervous system, MeHg is also toxic to other cell types/systems, including the immune system. For example, previous studies in animal models suggest that MeHg stimulates the immune system by inducing the release of interleukin (IL)-6 from murine glial cells,6) and by increasing IL-4 production and immunoglobulin (Ig)E levels in human leucocytes.7) By contrast, data indicate that MeHg inhibits the immune system in rat pups (exposed via the placenta and milk) by reducing the activity of natural killer cells,8) suppressing histamine release from rat mast cells,9) and reducing T cell numbers via induction of apoptosis.10) Despite several studies have evaluated the immunomodulatory effects of MeHg, the issue remains controversial. It is note worthy that many of these studies implicate relationship between MeHg and T-helper 2 (Th2) allegic response. However, there is very little information about the effects of MeHg exposure especially through a daily food intake on allergic disease patients. Thus, in the present study, we examined the effects of low-dose MeHg in a Th2 allergic response model, in which mice were immunized with antigen via the oral or subcutaneous routes and then exposed to MeHg. We found that low levels of MeHg exposure had no adverse effects on the Th2 immune response in mice immunized with either tested antigen.
Seven-week-old specific pathogen-free male BALB/c mice and 8-week-old male NC/Nga mice were purchased from Japan SLC (Shizuoka, Japan). Mice were allowed to acclimatize for 7 d upon arrival before being randomly divided into experimental groups. Mice were housed in an animal facility that was maintained at 24–26°C with 55–75% humidity under a 12 h light/dark cycle. Animals were provided with food (Crea Japan, Tokyo, Japan) and water. All experimental procedures used in this study conformed to the Procedures for Animal Experiments and were approved by the Committee for Animal Experiments at Kitasato University and the Guiding Principles for the Care and Use of Laboratory Animals.
Oral Immunization with Ovalbumin (OVA)BALB/c mice were divided into control and immunized groups. The immunized group was injected intraperitoneally with 100 µg of OVA (Sigma, MO, U.S.A.) on Days 1 and 14, respectively, according to the vaccination protocol described by Song et al.11) OVA was pre-mixed with aluminum hydroxide (2 mg; Sigma) in a total volume of 0.2 mL phosphate-buffered saline (PBS). Next, on Days 28, 30, 32, 35, 37, and 39, the mice were orally administered OVA (10 mg). MeHg (0, 0.02, or 1.0 mg·kg−1·d−1; Tokyo Chemical Industry, Tokyo, Japan) was administered five times per week for 6 weeks after the first OVA injection (Fig. 1A). The control group was injected with PBS instead of OVA and MeHg. The body weight of the animals was measured once a week. Twenty-four hours after the last MeHg treatment, the animals were sacrificed, and serum immunoglobulin and cytokine levels were measured.
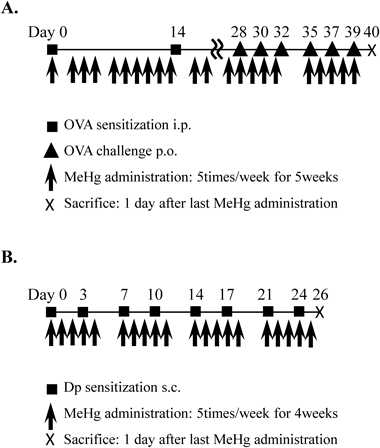
(A) Protocol 1: Mice were sensitized by intraperitoneal injection of OVA on Days 0 and 14 (squares). Intragastric challenge with OVA was then performed on Days 28, 30, 32, 35, 37, and 39 (triangles). MeHg (0, 0.02, or 1.0 mg·kg−1·d−1) was administered five times a week for 6 weeks, beginning after the first OVA injection (arrows). The control group received PBS instead of OVA and MeHg. Mice were sacrificed 1 d after the final MeHg administration. (B) Protocol 2: Mice were sensitized by subcutaneous injection of Dp on Days 0, 3, 7, 10, 14, 17, 21, and 24 (squares). MeHg (0, 0.02, 1.0, or 5.0 mg·kg−1·d−1) was then administered five times a week for 4 weeks, beginning after the first Dp injection (arrows). The control group received PBS instead of Dp and MeHg. Mice were sacrificed 1 d after the final MeHg administration.
NC/Nga mice were divided into control and immunized groups. The immunized group was injected subcutaneously (on the back) with 5 µg of mite extract [Dermatophagoides pteronyssinus (Dp); Cosmo Bio, Tokyo, Japan] in 200 µL of PBS. Injections were performed twice per week for 4 weeks. MeHg (0, 0.02, 1.0, or 5.0 mg·kg−1·d−1) was administered five times per week for 4 weeks following the initial Dp treatment (Fig. 1B). The control group received PBS instead of Dp and MeHg. Body weight was measured once a week. Twenty-four hours after the last injection of MeHg, the animals were sacrificed, and serum immunoglobulin and cytokine levels were measured.
Preparation of Total Brain HomogenatesBrains were excised, weighed, flash frozen in liquid nitrogen, and stored at −80°C until required for further processing. Frozen brain tissues were homogenized in 3 mL of 10 mM potassium phosphate buffer (pH 7.4) containing 0.1 mM ethylenediaminetetraacetic acid (Sigma-Aldrich, St. Louis, MO, U.S.A.), 0.1 mM phenylmethanesulfonyl fluoride (Nacalai Tesque, Kyoto, Japan), 1 µM pepstatin A (Peptide Institute, Osaka, Japan), and 2 µM leupeptin (Peptide Institute). The homogenates were then centrifuged at 105000×g for 1 h. The supernatants were stored at −80°C until analysis by enzyme linked immunosorbent assay (ELISA).
Quantitation of Serum Immunoglobulin and Cytokine Levels by ELISABlood samples were collected and allowed to clot for 2 h at room temperature. Samples were then centrifuged at 2500×g for 30 min and serum stored at −80°C. Brains were weighed and flash frozen in liquid nitrogen, and stored at −80°C until required for further processing as described above. ELISAs to measure OVA-specific IgE (OVA-IgE; Shibayagi, Gunma, Japan), OVA-specific IgG1 (OVA-IgG1; Shibayagi), total IgE (GenWay, San Diego, CA, U.S.A.), total IgG (GenWay), interferon (IFN)-γ (Endogen, Cambridge, MA, U.S.A.), IL-4 (R&D Systems, Minneapolis, MN, U.S.A.), and IL-13 (R&D Systems) in serum and in brain homogenates were then performed, according to the manufacturer’s instructions. The absorbance was read in a microplate reader at 450 nm. The control reading at 550 nm was then subtracted. The ELISA results were converted to U/mL (OVA-IgE), mU/mL (OVA-IgG1), ng/mL (total IgE), µg/mL (total IgG), and pg/mL (IFN-γ, IL-4, and IL-13), using values obtained from standard curves generated by assay of varying concentrations of recombinant OVA-IgE, OVA-IgG1, total IgE, total IgG, IFN-γ, IL-4, and IL-13.
Statistical AnalysisData sets were reported as the mean±standard error (S.E.) in Figs. 2, 3A, 4A, and 5A. Each value was plotted and the mean was expressed as line in Figs. 3B, C, 4B, C, 5B, and C. Differences between groups were determined by ANOVA. If the differences were significant, Dunnett’s test was used to compare each treatment group. A p value of <0.05 was considered statistically significant.
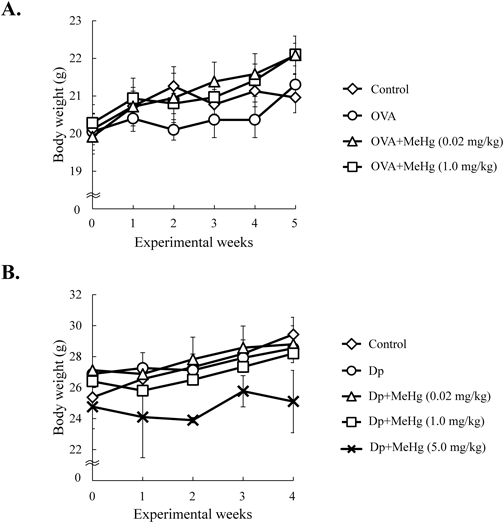
The body weight of mice treated with OVA (A) and Dp (B) was measured weekly. Results are expressed as the mean±S.E. (n=4–6).

(A) Serum levels of OVA-IgE, OVA-IgG1, IL-4, IL-13, and IFN-γ were measured in control (OVA−) and OVA-challenged mice (OVA+). Results are expressed as the mean±S.E. (n=5–6). * p<0.05, ** p<0.01. (B) Serum levels of OVA-IgE and OVA-IgG1 were measured in MeHg-treated mice. Each symbol represents an individual mouse. Line represents the mean of each group. (C) Serum levels of IL-4, IL-13, and IFN-γ were measured in MeHg-treated mice. Each symbol represents an individual mouse. Line represents the mean of each group. ND: not detected.

(A) Serum levels of total IgE, total IgG, IL-4, IL-13, and IFN-γ were measured in control (Dp−) and Dp-challenged mice (Dp+). Results are expressed as the mean±S.E. (n=4–6). * p<0.05, ** p<0.01. (B) Serum levels of total IgE and total IgG were measured in MeHg-treated mice. Each symbol represents an individual mouse. Line represents the mean of each group. (C) Serum levels of IL-4, IL-13, and IFN-γ were measured in MeHg-treated mice. Each symbol represents an individual mouse. Line represents the mean of each group. ND: not detected.

(A) The levels of total IgE, total IgG, IL-4, IL-13, and IFN-γ in the brain were measured in control (Dp−) and Dp-challenged mice (Dp+). Results are expressed as the mean±S.E. (n=4–6). * p<0.05. (B) Levels of total IgE and total IgG in the brains of MeHg-treated mice. Each symbol represents an individual mouse. Line represents the mean of each group. (C) Levels of IL-4, IL-13, and IFN-γ in the brains of MeHg-treated mice. Each symbol represents an individual mouse. Line represents the mean of each group. ND: not detected.
To examine the effects of a low-dose of MeHg on the Th2 immune response, we treated mice with a low-dose (0.02 mg·kg−1·d−1) (a dose roughly equivalent to the levels to which humans are exposed after consuming certain species of fish), intermediate-dose (1.0 mg·kg−1·d−1), or high-dose (5.0 mg·kg−1·d−1) of MeHg plus either OVA or mite Dp extract (Fig. 1). The body weight of each mouse was measured once a week throughout the administration period (Fig. 2). There was no significant difference between the body weight of mice treated with low- and intermediate-doses of MeHg and that of control mice treated with OVA (Fig. 2A) or Dp alone (Fig. 2B). However, mice treated with a high-dose of MeHg plus Dp for 1–5 weeks weighed slightly less than non-treated mice and mice treated with low- and intermediate-doses of MeHg (Fig. 2B). Of note, treatment with a high-dose of MeHg plus OVA had a lethal effect; therefore, this regimen was abandoned (data not shown).
To confirm the effects of MeHg on Th2 immune responses, we first performed an immunization experiment by injecting mice with OVA and then measuring the levels of allergy-related immunoglobulins and cytokines in the serum (Fig. 3). Serum levels of OVA-IgE, OVA-IgG1, IL-4, and IL-13 in MeHg-untreated OVA-immunization mice were significantly higher than those in the control group. However, no IFN-γ was detected in either group (Fig. 3A). Administration of a low- or intermediate-dose of MeHg plus OVA resulted in similar levels of serum OVA-IgE and OVA-IgG1 compared with those in the MeHg-untreated mice (Fig. 3B). Moreover, MeHg administration had no effect on IL-4 and IL-13 levels when compared with those in the MeHg-untreated mice (Fig. 3C). By contrast, serum IFN-γ levels increased in some mice treated with intermediate-dose of MeHg (Fig. 3C).
To confirm the effects of MeHg on Th2 immune responses, we next injected mice with Dp (subcutaneously) (Fig. 4). Serum levels of total IgE, total IgG, and IL-13 in MeHg-untreated Dp-immunized mice were significantly higher than those in the control group. However, no IL-4 or IFN-γ was detected in either group (Fig. 4A). In the Dp-treated mice, MeHg administration had no effect on total IgE and total IgG levels when compared with those in the MeHg-untreated mice (Fig. 4B). Furthermore, no changes in IL-13 levels were observed following MeHg administration (Fig. 4C). Levels of total IgE, total IgG, and IL-13 in the brains of Dp-treated mice were significantly higher than in those of control mice (Fig. 5A); moreover, MeHg administration to the immunized mice had no effect on total IgE, total IgG, IL-13 and IFN-γ levels when compared with those in the MeHg-untreated mice in the brains (Figs. 5B, C).
The present study examined the effects of low-dose MeHg on the immune response of mice. The MeHg dose applied in this study was designed as to the possible MeHg exposure derived from the daily consumption of certain fish species. The study was based on the two mouse models, both of which display enhanced Th2 immune responses against antigens. In the first model, mice were sensitized to OVA via oral administration, thereby mimicking food allergy-like symptoms, leading to increased expression of IgE, IgG1, IL-4, and IL-13, which are typical markers of the Th2 immune response.11) In the second model, male NC/Nga mice were immunized with a mite antigen to mimic allergic dermatitis. These mice also showed significant increases in the levels of serum IgE, IgG1, and IL-13.12,13) The increased serum levels of Th2-type cytokines and antibodies, including IgE, IgG, IL-13, and/or IL-4, in the immunized groups (Figs. 3A, 4A) suggest that a Th2 allergic response was elicited in both experiments. Importantly, MeHg administration to immunized mice led to either a slight dose-dependent decrease or to unchanged levels of Th2 markers in the serum, including OVA-IgE, OVA-IgG1, IL-4, and IL-13 (OVA-immunized mice; Figs. 3B, C) and total IgE and IL-13 (Dp-immunized mice; Figs. 4B, C). In addition, a marked increase in serum IFN-γ levels, which inhibits Th2 immune responses,14) was observed in several mice following treatment with 1.0 mg·kg−1·d−1 MeHg (Fig. 3C). This result is consistent with the suppression of Th2 immune responses. Moreover, MeHg administration to immunized mice led to either a slight dose-dependent reduction or no change in the levels of total IgE, total IgG, and IL-13 in the brain (Fig. 5). Taken together, the findings from the two models suggest that MeHg has negligible adverse effects on Th2 responses.
Abnormal immune conditions, such as those associated with autoimmune and allergic diseases, can enhance immune reactions to environmental chemicals. For example, bisphenol A increases autoantibody production in mice with systemic lupus erythematosus,15) and phthalate ester increases serum IgE levels.16) Diesel exhaust particles (DEP), derived from major constituents of atmospheric particulate matter in industrialized countries, have adverse health effects in the context of their immunotoxic potential.17–19) In addition, the adverse effects of DEP can be clearly seen in the atopy-prone milieu.20) Furthermore, our previous studies in vitro suggest that DEP induce cytokine production by splenocytes isolated from atopy-prone NC/Nga mice.21,22) By contrast, effects of MeHg on immune system have been still elusive. However, our results presented here suggest that MeHg is unlikely to increase immune responses in mice with a dysregulated immune system. The target/mechanism underlying immunoactivation or immunosuppression by MeHg is unclear, but one possibility is that MeHg impairs the activation or differntiation of immune cells such as dendritic cells, lymphocytes.
To our knowledge, our study is the first examination of the effect of low dose MeHg exposure on Th2 immune responses in antigen-immunized mice. We demonstrate that exposure to low doses of MeHg is not relevent to increasing a risk of dysregulated Th2 immune responses. Thus, we suggest that low doses of MeHg do not have aggravating effects on allergies and atopic dermatitis. Based on our results, we propose that seafood consumption which is a main source of MeHg intake through the diet may not affect the condition of Th2 type allergic patients. Further researches are needed to reveal more details of MeHg effects on the immune system at the cellular and molecular levels.
We thank Miss H. Abe, Mr. M. Itoh, Mr. Y. Kondo, Mr. R. Nakagawa, Miss S. Nakayama, Mr. N. Kida, Mr. T. Tsuboi, Mr. T. Nishiyama, and Miss M. Maruya for technical assistance. This work was supported in part by a Grant-in-Aid for Young Scientists (B) (No. 15K18887) to R. Nakamura from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
The authors declare no conflict of interest.