2017 年 40 巻 12 号 p. 2075-2080
2017 年 40 巻 12 号 p. 2075-2080
Chondroitin sulfate (CS) is a sulfated polysaccharide produced by chondrocytes. Alkaline phosphatase (ALP) is an important enzyme involved in the mineralization of chondrocytes. In recent years, it has been reported that CS regulates the differentiation of various cells. In this study, we investigated the effect of supplemented CS on ALP activity and mineralization of the chondrogenic cell line, ATDC5. In addition, hyaluronic acid (HA), a non-sulfated and acidic polysaccharide, was used in comparison to CS. CS and HA significantly suppressed ALP activity without affecting ATDC5 cell proliferation. In addition, although the inhibition of ALP activity was observed at every time point, Alp mRNA expression level was not affected by CS. The suppressive effect of CS on ALP activity was abrogated by pre-treatment with chondroitinase ABC (CSase). CS and L-homoarginine (hArg), an inhibitor of ALP, significantly suppressed mineralization in ATDC5 cells. In conclusion, supplemented CS directly inhibits ALP to prevent the progression of chondrocytes from differentiation to mineralization.
Chondroitin sulfate (CS), a glycosaminoglycan (GAG), is widely present intravitally. CS is a polysaccharide composed of a repeated unit of disaccharide, glucuronic acid, and N-acetylgalactosamine modified with a sulfate group. Hyaluronic acid (HA), also a type of GAG, is a polysaccharide of glucuronic acid, and N-acetylglucosamine. CS and HA are present in the extracellular matrix (ECM) of various organs and tissues including the brain, skin and cartilage.1,2)
Articular cartilage consists of chondrocytes, elastin, collagen, and a large amount of ECM abundant in CS and HA.2) Chondrocytes undergo a differentiation process called endochondral mineralization, where the cells are finally transformed into calcified chondrocytes via proliferation, maturation, and hypertrophy.3) Chondrocytes in the growth plate cartilage differentiate during the process of endochondral mineralization.4) However, most of the chondrocytes present in the articular cartilage do not differentiate.5)
In a healthy state, chondrocytes in the articular cartilage produce aggrecan, a large proteoglycan formed by numerous CS, keratan sulfate, and core protein.6,7) It is present in the ECM in the form of proteoglycan aggregates, in which many aggrecan molecules interact with HA and a linker protein to stabilize each interaction.2,8–10) Because the cartilage ECM, rich in GAGs such as CS and HA, exists to cover chondrocytes, GAGs are considered to play various regulatory roles in the proliferation and differentiation of chondrocytes. Over-sulfated CS such as CS-E and high-molecular-weight HA have been recently reported to promote the differentiation of chondrocytes.11,12) Although the mineralization of chondrocytes is essential for osteogenesis, ectopic mineralization in articular cartilage induced by hypertrophic chondrocytes in patients with osteoarthritis (OA) causes cartilage degeneration.13–16) Alkaline phosphatase (ALP) is located on the cell surface of chondrocytes and hydrolyzes pyrophosphoric acid to inorganic phosphoric acid, thereby supplying the substrate necessary for mineralization.17–20) The inorganic phosphoric acid taken into chondrocytes via a phosphate transporter binds to calcium present in the cells to form calcium phosphate crystals; this process is termed mineralization.21) ALP expression and activity in chondrocytes are low in the proliferating stage, increasing rapidly at the mature stage, and remaining high thereafter.17) Therefore, ALP could be a trigger of endochondral mineralization and, thus, a marker of the initial differentiation.
Because ALP is located outside the chondrocytes, the large amounts of CS and HA that exist around chondrocytes as the ECM might change ALP activity, altering the differentiation of the chondrocytes. In this study, we examined the effects of supplemented CS and HA on ALP in the chondrogenic cell line, ATDC5. ATDC5 is a progenitor chodrogenic cell line that reproduces endochondral ossification.22) This cell line sequentially differentiates from proliferative chondrocyte to mature chondrocyte, hypertrophic chondrocyte, and calcified chondrocyte. Meanwhile, the effect of supplemented CS and HA on the calcification of ATDC5 cells was evaluated.
CS from shark cartilage (molecular weight (MW)=31000) and HA from chick cartilage (MW=100000) were kindly provided by Zeria Pharmaceutical Co., Ltd. (Tokyo, Japan). Chondroitinase ABC (CSase) was purchased from Sigma-Aldrich Co., Ltd. (St. Louis, MO, U.S.A.). L-Homoarginine (hArg), a bone ALP inhibitor, was purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Low-molecular-weight CS was prepared by digesting CS with CSase: 10 mg/mL aqueous CS and CSase (final concentration, 5 mU) was incubated for 0.25, 0.5, 0.75, 1, 2, 4 or 24 h at 37°C.
Cell CultureA chondrogenic cell line, ATDC5, was obtained from Riken Cell Bank (Ibaraki, Japan). The ATDC5 cells were cultured in Dulbecco’s modified Eagle medium/Ham’s F12 (DMEM/F12) medium (Life Technologies Japan, Tokyo, Japan) supplemented with 5% FBS (Sigma-Aldrich) and penicillin (100 µg/mL)-streptomycin (50 µg/mL)-kanamycin (50 µg/mL) (Meiji Seika, Tokyo, Japan). The detail of culture conditions was described in previous reports.23,24) The cell cultures were maintained at 37°C in a humidified atmosphere of 5% CO2.
WST-1 AssayATDC5 cells were seeded in a 96-well plate at approximately 3×103 cells per well. After 1 d of culture, CS or HA was added to the cells at a final concentration of 1–1000 µg/mL. The cells were cultured for an additional day. Subsequently, the medium was replaced with fresh medium containing 10% WST-1 reagent (Roche, Basel, Switzerland) and incubated for 4 h at 37°C. The absorbance at a wavelength of 440 nm was measured using a Spectra Max M2e system (Molecular Devices Japan, Tokyo, Japan).
ALP Activity StainingATDC5 cells were seeded in a 96-well plate at approximately 5×103 cells per well. After 1 d of culture, CS or HA at a final concentration of 1–1000 µg/mL or 10 mM hArg was added to the cells. The cells were cultured for 0, 4, 7, and 14 d. Next, the cells were fixed with 20% formalin for 20 min and then rinsed with water for 15 min at room temperature. The cells were incubated with a staining solution, i.e., 0.05 M 2-amino-2-methyl-1-propanol (AMP) (Nacalai Tesque, Kyoto, Japan) buffer (pH 9.8) containing 10 mM naphthol AS-BI phosphate (Sigma-Aldrich) and 1 mM Fast Red violet LB salt (Sigma-Aldrich) for 20 min at 37°C. The staining solution was removed and the cells were washed with water. The ALP stained areas were scanned using an image scanner and analyzed qualitatively using Image J (National Institutes of Health, Bethesda, MD, U.S.A.). In the case of CSase treatment, 5 mU of the enzyme was added to the cells 12 h before the staining.
Alcian Blue (AB) StainingATDC5 cells were seeded in a 96-well plate at approximately 5×103 cells per well. After 1 d of culture, CS or HA was added to the cells at the final concentration of 1000 µg/mL. The cells were cultured for 4 d. Next, the cells were treated with 5 mU of CSase overnight and then the cells were stained with AB. The cells were fixed with 20% formalin for 20 min and then washed with 0.1 M hydrochloric acid (Wako Pure Chemical Industries, Ltd., Osaka, Japan) for 15 min. Next, the cells were reacted overnight with pH 1.0 AB solution (Muto Pure Chemicals, Tokyo, Japan) to stain sulfated GAG. The staining solution was removed and the cells were rapidly rinsed with 0.1 M hydrochloric acid. The AB-stained areas were scanned using an image scanner and analyzed qualitatively using Image J. In the case of CSase treatment, 5 mU of the enzyme was added to the cells 12 h before the staining.
Alizarin Red (AR) StainingATDC5 cells were seeded on a 24-well plate at approximately 3×104 cells per well. CS or HA at a final concentration of 1000 µg/mL, or 10 mM hArg was added to the cells after 1 d of culture. After an additional 5 d of culture, the medium was replaced with insulin-transferrin-sodium selenite (ITS, Sigma-Aldrich)-supplemented medium. The cells were maintained and the medium was replaced every day for 9 d. The control cells added ITS were confirmed to be AB staining was positive and some cartilage differentiation marker (Sox9, Runx2, Col2 and Aggrecan) mRNA are expressed at this stage. Next, the cells were fixed with 20% formalin for 20 min and then rinsed with water before 1% AR (Sigma-Aldrich) solution (pH 6.3) was added to the cells. The staining solution was removed and the cells were rinsed with water. The AR stained areas were scanned using an image scanner and analyzed qualitatively using Image J software.
RT-PCRATDC5 cells were cultured in a 6-cm dish at approximately 2×105 cells per dish. After 1 d of culture, CS or HA was added to the cells at the final concentration of 1000 µg/mL. The cells were cultured for 0, 4, 7, and 14 d. Next, total RNA was extracted from the cells using RNAiso plus reagent (TaKaRa Bio, Tokyo, Japan) according to the manufacturer’s instructions. cDNA was prepared from 1 µg of total RNA using the Superscript First-Standard Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA, U.S.A.). Amplification of cDNA was performed using EX Taq HS (TaKaRa Bio). The primer sequences and PCR conditions are shown in Table 1, except for the initial denaturation at 94°C for 30 s. The PCR products were electrophoresed on a 1.5% agarose gel (TaKaRa Bio) and visualized with ethidium bromide (Sigma-Aldrich). All gels were digitally imaged and analyzed using a Gel Doc™ EZ Imager (BIO RAD, Hercules, CA, U.S.A.). The signals were normalized to those of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) transcripts.
| Gene | Sequences (5′→3′) | PCR condition | |||
|---|---|---|---|---|---|
| Annealing (°C/s) | Extension (°C/m) | Cycles | |||
| Alp | Forward | ACATCATGAGGGTAAGGCCAAG | 58/30 | 72/3 | 20 |
| Reverse | TGGGCCTGGTAGTTGTTGTGAG | ||||
| Gapdh | Forward | TTGACCTCAACTACATGG | |||
| Reverse | TGAGGTCCACCACCCTG | ||||
Data are presented as the mean±standard error (S.E.). Statistical analysis of differences was performed using Dunnett’s or Tukey’s multiple comparisons test. Statistical analysis was performed using StatMate 3 (ATOMUS Inc., Tokyo, Japan).
First, we investigated the effect of supplemented CS and HA on the proliferation of ATDC5 cells using the WST-1 assay. CS and HA did not affect ATDC5 cell proliferation up to the highest concentration used (1000 µg/mL). Next, the effect of supplemented CS and HA on the ALP activity of ATDC5 cells cultured in the presence of CS or HA for 4 d was evaluated by staining for ALP activity. Although, CS and HA did not affect ALP activity at 1–100 µg/mL, at a concentration of 1000 µg/mL, both significantly suppressed ALP activity (Fig. 1). Therefore, we performed the subsequent experiments using 1000 µg/mL CS and HA.
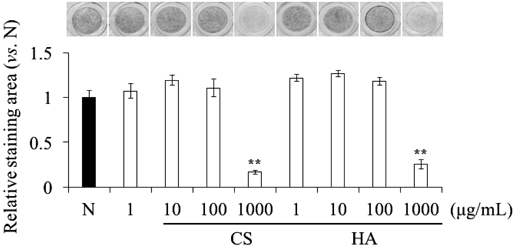
Data are the mean±S.E. (n=4). Significant differences vs. N are shown by ** p<0.01 using Dunnett’s test.
Because high-concentration of CS and HA significantly suppressed ALP activity in ATDC5 cells for 4 d, we investigated the long-term effect of supplemented CS and HA on ALP activity. At 7 and 14 d, CS and HA significantly suppressed ALP activity (Fig. 2). Because the ALP activity started to increase in the early differentiation stage of ATDC5 cells, we evaluated the ALP activity in cells cultured for 4 d in the subsequent experiments.
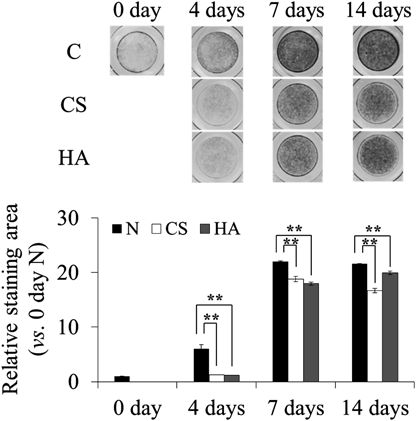
Upper photos are representative of ALP activity staining. The graph shows the relative staining area intensity. Data are the mean±S.E. (n=4). Significant differences vs. N are shown by ** p<0.01 using Dunnett’s test.
Figure 3 shows the expression of Alp mRNA in ATDC5 cells cultured with or without supplemented CS or HA for 0–14 d. The expression of Alp mRNA in all groups increased over time. However, CS and HA did not show altered expression when compared to control cells (Figs. 3a, b).

(a) The photos are representative of the PCR products stained by ethidium bromide in the agarose gel. (b) Alp mRNA levels were normalized against Gapdh mRNA levels and then compared to N at day 0. Data are the mean±S.E. (n=3). No significant difference vs. N at each day was observed (Dunnett’s test).
To investigate the effect of the molecular size of CS on ALP activity in ATDC5 cells, we prepared low-molecular-weight CS digested by CSase. As shown in Fig. 4a, CS was gradually digested into smaller molecules depending on the CSase treatment time. No staining appeared after more than 4 h of CSase treatment. Treatment with the moderately digested CS for 4 d significantly suppressed ALP activity in ATDC5 cells similarly to intact CS (Fig. 4b). In contrast, the completely digested CS did not affect ALP activity.

CS was treated with or without CSase to prepare low-molecular-weight CS, and the effect of fragmentation of CS on ALP activity was investigated. (a) CS digested by CSase was visualized using 0.05% toluidine blue in a 1% agarose gel. (b) ALP staining of ATDC5 cells cultured with digested CS. The cells were treated with CS digested by CSase for 4 d. Data are the mean±S.E. (n=4). Significant differences vs. N are shown by ** p<0.01 using Dunnett’s test.
We attempted to pre-treat ATDC5 cells with CSase before ALP staining. The suppressive effect of CS and HA on ALP activity was significantly abrogated by CSase pre-treatment (Fig. 5a). Furthermore, we performed AB staining (pH 1.0 or 2.5) to detect GAG on the cells. As shown in Fig. 5b, the cells cultured with CS were a blue color, indicating accumulation of GAG. However, the GAG accumulation did not appear with CSase pre-treatment.
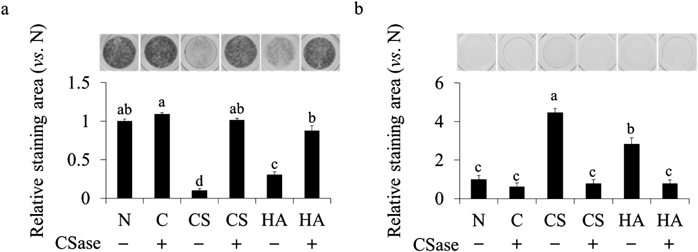
The cells were cultured with CS and HA for 4 d. At 4 d, 5 mU of CSase was added to the cells and reacted with CS or HA overnight. The cells cultured with CS, HA, and/or CSase were evaluated by ALP activity staining (a) and pH 1.0 or 2.5 AB staining (b). Data are the mean±S.E. (n=4). Different letters indicate significant differences (p<0.01, Tukey’s test).
Finally, we examined the effect of ALP inhibition on mineralization of ATDC5 cells. hArg, an ALP inhibitor, significantly suppressed ALP activity in ATDC5 cells similarly as CS and HA (Fig. 6a). The control cells cultured with ITS formed remarkable nodules that were stained by AR. Supplemented CS, HA, and hArg significantly suppressed the mineralization of ATDC5 cells compared with the control (Fig. 6b).

The cells were cultured with CS, HA, and hArg for 15 d in the presence of ITS supplement. (a) The effect of CS, HA, and hArg on ALP was determined by ALP staining. (b) The effect of CS, HA, and hArg on mineralization was determined by AR staining. Data are the mean±S.E. (n=6, 4, respectively). Significant differences vs. N are shown by ** p<0.01 using Dunnett’s test (a), and p<0.001 using Tukey’s test (b).
ECM in articular cartilage contains a large amount of CS and HA, which possesses high water retention capacity.2) Thus, the articular cartilage that abounds with ECM lubricates the surface of the articulation to prevent abrasion. Recent studies reported that CS and HA increased GAG production and promoted the differentiation of chondrocytes.11,12,25–27) However, the effect of CS and HA on ALP activation and mineralization, which are crucial events in the differentiation process of chondrocytes, is unclear. In this study, we investigated the effect of supplemented CS and HA on ALP activity and mineralization using a chondrogenic cell line, ATDC5.
ALP activity can be evaluated without the inducer. Our data revealed that supplemented CS and HA significantly suppressed ALP activity without affecting cell proliferation of early stage chondrocyte differentiation in ATDC5 (Fig. 1). The suppression of ALP activity by CS lasted even for a long period (Fig. 2). However, the suppressive effect was observed at the highest concentration (1000 µg/mL) of CS and HA (Fig. 1). We did not supply any inducers for progressing differentiation to the cells because ALP activity had been observed enough without insulin.23,24,28) Additionally, unintentional inhibition of ALP could be occurred by insulin.29)
In the articular cartilage and growth plate, chondrocytes are covered with a large amount of ECM, including CS and HA. It is possible that excess CS and HA around chondrocytes affect ALP activity and thus regulate the differentiation of chondrocytes in vivo. However, we found that the expression level of Alp mRNA was not affected by addition of CS and HA (Fig. 3). A progressive increase in ALP activity has been observed during the differentiation of chondrocytes in the articular cartilage, whereas low activity was observed in the proliferative stage, higher activity in the maturing stage, and the highest activity in the hypertrophic stage.17,30) In the present study, ALP protein appeared to be normally produced at all stages even in the presence of excess of CS and HA. The excess CS and HA probably interfered with the activity of ALP protein expressed on the cell membrane of ATDC5 cells.
In the present study, the suppressive effects of supplemented CS and HA on ALP activity were prevented by pre-treatment with CSase (Fig. 5a). Additionally, continuous addition of high-concentration CS and HA to ATDC5 cells resulted in a higher amount of GAG on ATDC5 cells (Fig. 5b). Although endogenous GAG levels were undetectably low in the early differentiation stage of ATDC5 cells, a remarkable amount of GAG was detected upon exogenous addition of CS and HA. This result suggests that the additional high-concentration CS and HA accumulated on the cells. CSase initially hydrolyzes CS into a smaller molecule, then completely degrades it to a conjugated disaccharide.31) The completely hydrolyzed CS prepared in this study had no effect on ALP activity (Fig. 4). These results suggest that CS accumulated around the chondrocytes directly suppresses ALP activity on the cell surface. The pericellular environment of ATDC5 cells might be acidified by the accumulated CS and HA, thereby possibly affecting the ALP activity. Several studies have revealed that CS interacts with proteins expressed on the cell surface and regulates their function.32,33) For example, CS suppresses the differentiation of osteoclasts by interacting with proteins on the osteoclast surface.32) In addition, CS promotes the differentiation of osteoblasts by interacting with N-cadherin on the osteoblast surface.33) We demonstrated that supplemented CS and HA suppressed the mineralization of ATDC5 cells (Fig. 6b), as did hArg, an ALP inhibitor. Hydrolysis of pyrophosphate to inorganic phosphate by ALP underlies the mineralization of chondrocytes.34–36) Our data suggest that CS and HA regulate the differentiation, i.e., mineralization of chondrocytes via direct suppression of ALP activity. Such control cells with ITS were confirmed to be AB-positive and some cartilage differentiation gene markers (Sox9, Runx2, Col2 and Aggrecan) of the cell were enhanced at this stage (data not shown). Further experiment using chondroitinase ABC will be necessary to clarify the regulatory roles of endogenous CS and HA on ALP activity in the ATDC5.
In the articular cartilage of healthy individuals, most chondrocytes are mature chondrocytes.37) The mature chondrocytes produce aggrecan, a complex of sulfated polysaccharides such as CS and core protein, which plays an important role in mediating chondrocyte-chondrocyte and chondrocyte-matrix interactions through its ability to bind to HA.10) A decrease in the amount of ECM, hypertrophic differentiation of mature chondrocytes and ectopic mineralization have been observed in the articular cartilage of patients with OA.13–15,38–40) Therefore, the ectopic mineralization in OA likely at least partly results from abrogation of the suppressive effect of ALP activity by decreasing the amount of ECM around the chondrocytes.
In conclusion, we identify a new role of supplemented CS in chondrogenic differentiation, that CS directly suppresses ALP activity to arrest chondrogenic differentiation. Our finding would be useful for the treatment for ectopic mineralization of chondrocytes. Further experiments are now in progress to clarify the interaction between CS and ALP in chondrocytes.
We thank Zeria Pharmaceutical Co., Ltd. for providing CS and HA for use in this study.
Masahiro Wada received a Research Grant from Zeria Pharmaceutical Co., Ltd.