2017 Volume 40 Issue 12 Pages 2039-2044
2017 Volume 40 Issue 12 Pages 2039-2044
Protease-activated receptor 2 (PAR2) is a G protein-coupled receptor activated by serine proteases released from tissues or by synthetic peptide ligands administered pharmacologically. Its wide expression in the cardiovascular system, particularly within the endothelium, vasodilation activity, and link to increased expression of inflammatory cytokines positions PAR2 as a potentially important regulator of vascular pathology under conditions of tissue inflammation, and injury; and thus, a pharmaceutical target for new therapeutics. Obesity is considered a chronic low-grade systemic inflammatory condition as inflammatory cytokines released from adipocytes are closely related to development of metabolic syndrome and related disorders. Our work over the past five-years has focused on the changes in vasomotor functions of PAR2 in metabolic syndrome, using an animal model known as the SHRSP.Z-Leprfa/IzmDmcr rats (SHRSP.ZF). In young SHRSP.ZF that had already developed impaired responses to nitric oxide, we reported that PAR2-induced endothelium-dependent vasodilation is preserved. However, this PAR2 vasodilation decreased with increasing age and further chronic exposure to the conditions of metabolism disorder. These findings raise the possibility that PAR2 regulates tissue perfusion and can protect organs from injury, which is an increasing clinical concern at later stages of metabolic syndrome. Here we present our studies on the time-dependent changes in vasoreactivity to PAR2 in metabolic syndrome and the underlying mechanisms. Furthermore, we discuss the implications of these age-related changes in PAR2 for the cardiovascular system in metabolic syndrome.
Vascular endothelial cells cover the inner wall of blood vessels and regulate the tonus of the underlying vascular smooth muscle cells by releasing of vasodilator and vasoconstrictor factors.1) The first evidence for the endothelium-dependent relaxing factors (EDRFs) was the discover that acetylcholine can induce vasodilation by production of endothelial nitric oxide (NO) through activating muscarinic receptor on endothelial cells.2) In the past several decades, researchers investigated the roles of the vascular endothelium in the cardiovascular system, and also determined that the endothelium produces not only EDRFs but also endothelium-dependent contractile factors (EDCFs). In addition to the endothelium, blood cells and tissues, such as fat surrounding the blood vessel walls, also generate or carry factors that lead to endothelial dysfunction, i.e., decreases in production of EDRFs, reduced bioavailability of NO, or imbalance of production of EDRFs and EDCFs. Endothelial dysfunction is a contributing mechanism to the clinical expression and progression of vascular diseases, including hypertension, stroke, diabetes, atherosclerosis.3,4)
Protease-activated receptor 2 (PAR2) is expressed on the vascular endothelium, and when it is activated, causes endothelium-dependent vasodilations of a variety of arteries in human and experimental animals via releasing of NO, prostanoids, and endothelium-dependent hyperpolarizing factor (EDHF).5–7) PAR2 is a member of PAR, and it is a class-A G protein-coupled receptor (International Union of Basic and Clinical Pharmacology nomenclature site: http://www.guidetopharmacology.org/nomenclature.jsp). There are four members of the PARs receptor family.8) PAR1 and PAR4 are generally expressed on human platelets and activated by thrombin, and a PAR1 antagonist, vorapaxar, is now approved as anti-platelet drugs by Food and Drug Administration (FDA) in U.S.A.9,10) By contrast, as shown in Fig. 1, PAR2 is expressed in the tissues and cells of the cardiovascular, respiratory, nervous, and digestive systems. PAR2 is activated when its N-terminus is cleaved by serine proteases, e.g. trypsin, or by synthetic ligands, including PAR2-activating peptides and small molecules, e.g. 2-furoyl-LIGRLO-NH2 (2fLIGRLO) and SLIGRL-NH2.11–13) A recent paper suggests that thrombin could activate PAR2 in situations, where high levels of active thrombin can be generated, such as in a tumor microenvironment or in the setting of acute tissue trauma.14) Also, inflammatory stimuli, such as interleukin-1α and tumor necrosis factor-α,15) can induce expression of PAR2 in the endothelium, which can lead to increased release of cytokines.
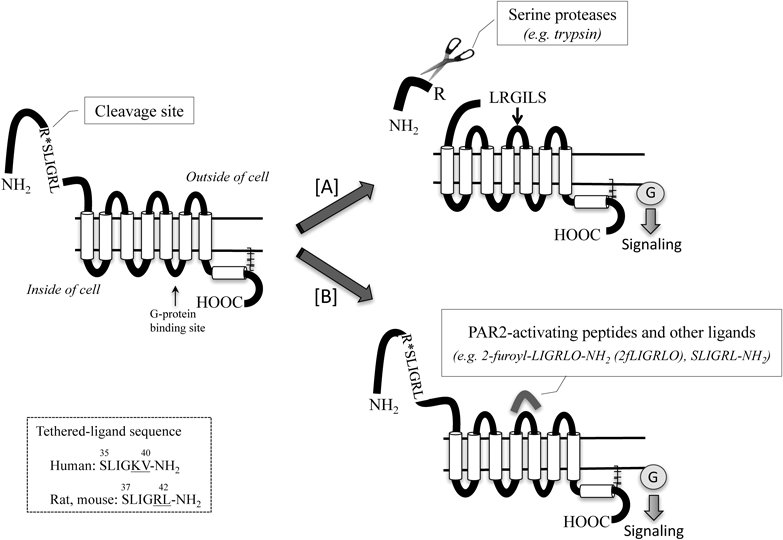
[A] PAR2 is cleaved by serine protease (trypsin, tryptase, coagulation factor VIIa and Xa) at specific site within the N-terminus, and unmasking tethered ligands bind to the second extracellular loops, resulting in the activation. [B] Alternatively synthetic peptide also known as PAR2-activating peptide, mimics the tethered ligand, bind within the second extracellular loop to activate the receptor. Peptide sequences are listed using the standard one-letter abbreviations for each amino acid.
PAR2 expression is increased in the adipose tissue of obese humans and rats. This higher level of PAR2 expression is proposed to contribute to the development of inflammation and metabolic disorders in diet-induced obese rats.16) Since inflammatory cytokines released from adipocytes are closely associated with the development of insulin resistance and cardiovascular disease, metabolic syndrome is referred as being a chronic low-grade systemic inflammatory conditions.17) The risk of cardiovascular disease and diabetes is raised by the cluster of cardiovascular risk factors (central obesity, abnormal plasma cholesterol and triglycerides levels, high plasma glucose levels, and increased blood pressure) that clinically characterize metabolic syndrome in humans.18,19) The prevalence of metabolic syndrome is increasing in the world, so treatments for reversal or prevention are needed urgently.18,19) Because of the increasing interest in PAR2 as a target of therapeutics, we recently conducted a systematic review of the roles played by PAR2 in obesity, diabetes, and metabolic syndrome, and highlighted current themes of investigation.20) For our own part, we have used an animal model of metabolic syndrome, SHRSP.Z-Leprfa/IzmDmcr rats (SHRSP.ZF), to investigate the time- and age-dependent changes that occur over the time–course of metabolic syndrome.21,22) Here in this review article, we summarize the findings from our studies of ageing/chronic exposure to metabolic abnormalities associated with metabolic syndrome on PAR2-mediated vasodilation in arteries of SHRSP.ZF. Furthermore, we discuss the role of PAR2 and the implications of its age-related changes for the circulatory function of the cardiovascular system in metabolic syndrome, and the potential for pharmaceutical development.
To investigate time-dependent changes in PAR2-mediated vasodilation in metabolic syndrome, we use an animal model, SHRSP.ZF. The SHRSP.ZF strain was established by crossing stroke-prone spontaneously hypertensive rats (SHRSP) and Zucker fatty rats (ZF).23) Young SHRSP.ZF (10–13 weeks of age) exhibit similar characteristics of metabolic syndrome observed in humans including a slowly worsening progression with age. In Japan, people are diagnosed with metabolic syndrome based on waist circumference, as a surrogate estimated measure of visceral fat, and two or more criteria as follows: high blood pressure, high fasting blood glucose, high serum triglycerides, or low high-density lipoprotein-cholesterol. Young SHRSP.ZF (13 weeks of age) already displayed the symptoms of the metabolic syndrome, e.g. approximately 1.2-times greater waist length to body length ratio, as an index of abdominal obesity, 1.2-times higher body weight, 1.2-times higher blood pressure, 8-times higher serum triglycerides, 1.6-times higher blood glucose than that of non-obese, normotensive control Wistar-Kyoto rats (WKY); also, these abnormalities are present at 18–23 weeks of age.21) Using the SHRSP.ZF with metabolic syndrome, we have examined aortas and mesenteric arteries to determine the endothelium-mediated vasodilator function of blood flow conductance- versus resistance-type arteries, respectively. Until 20 weeks of age, vasodilations in response to PAR2-activating peptide, 2fLIGRLO, are preserved even when NO mediated vasodilations (in response to nitroprusside) are attenuated in thoracic aortas and mesenteric arteries of SHRSP.ZF compared to that of control WKY.21,22) Increased levels of reactive oxygen species (ROS), e.g. superoxide, and decreased soluble guanylyl cyclase are described as some of the mechanisms that inhibit the ability of the vascular endothelium to affect NO-mediated vascular smooth muscle in diabetic humans and related models in animals. We reported that a maintained level of cyclic 3′,5′-guanosine monophosphate (cGMP) accumulation resulting from compensatory increased NO biosynthesis due to phosphorylation of Ser1177 in endothelial NO synthase (eNOS), contributed to the preserved PAR2-mediated vasodilations in aortas of SHRSP.ZF21) (Fig. 2). PAR2 is known to activate numerous protein kinase pathway that may be responsible for the phosphorylation of Ser1177 in eNOS, including AMP kinase, protein kinase A, calmodulin kinase II and Akt.24,25) In smaller caliber arteries (mesenteric arteries) of SHRSP.ZF, altering the distribution of endothelial derived relaxing factors, to increase NO versus other non-NO mediators, offset an apparent decrease in non-NO mediated vasodilation mechanisms.22)

Key findings supporting the idea that PAR2-activating peptide, 2-furoyl-LIGRLO-amide (2fLIGRLO), selectively increases phosphorylated endothelial nitric oxide synthase (phospho-Ser1177-eNOS) protein in aortas of SHRSP.ZF versus Wistar-Kyoto rats (WKY) (B). The data are summarized from Maruyama.21) Determination methods and conditions in detail are reported previously.21) Results are expressed as the mean±S.E.M. * p<0.05 by one-way ANOVA. n, number of animals. NO; nitric oxide, eNOS; endothelial NO synthase, sGC; soluble guanylyl cyclase, cGMP; cyclic GMP, ↑; increase in the expression of phospho-Ser1177-eNOS in response to 2fLIGRLO.
Preserved PAR2-mediated vasodilations have been reported from other groups using another animal model of metabolic syndrome and diabetes. Different mechanisms for the preservation are seen in each model and in arteries isolated from different anatomical sites. For example, enhancement of NO production via increase in PAR2 protein expression contributes to the enhanced PAR2-mediated vasodilation in mesenteric arteries of Goto–Kakizaki rats, type 2 diabetes model, at 32–40 weeks of age.26) In contrast, non-NO relaxing mechanisms, which includes endothelium-dependent hyperpolarization of vascular smooth muscle via endothelial calcium-activated potassium channels, contribute to the retention of PAR2-mediated vasodilations in mesenteric arteries of obese db/db mice at 12 weeks of age27) and in middle cerebral arteries of diet-induced obesity rats at 23–27 weeks of age.28) One group of researchers reported that PAR2-mediated vasodilations of aortas from non-obese diabetic mice (NOD) at 13–22 weeks of age29) became sensitive to inhibitors of cyclooxygenases, so inferring interactions between PAR2 and the vasculature exposed to inflammation conditions. In any case, PAR2-mediated vasodilations are preserved or enhanced, but are not impaired in metabolic abnormal conditions, even where the mediators of the smooth muscle relaxing pathways may differ between conductance and resistance-type arteries.
To our knowledge, the only other researchers to have specifically examined age as a factor in a model of metabolic dysfunction with regards to PAR2, studied the aortas from non-obese diabetic (NOD) mice.29) In that study, the researchers reported the severity of glycosuria increased with age and the aortas from the oldest NOD animals were most sensitive to PAR2 agonist. The diabetes and metabolic dysfunction associated with the NOD mouse strain is one of many used for research on type 1 diabetes in humans. Causes of and treatments for type 2 diabetes differs from type 1 diabetes although many of the features of the vascular and cardiac complications are similar. The age dependent progression of metabolic dysfunction in SHRSP.ZF resembles type 2 diabetes in humans.
In contrast to the study in NOD mice, we find that PAR2’s functional vascular responses and its expression are decreased by ageing and chronic in-vivo exposures to metabolic abnormalities in SHRSP.ZF (Fig. 3A). That is, in SHRSP.ZF at 30 weeks of age, PAR2-mediated vasodilation is impaired as similar to nitroprusside in thoracic aortas and mesenteric arteries, and the impaired relaxation is correlated with decreases in PAR2 mRNA or protein expressions in aortas30) (Fig. 3B). We therefore focused on relationship among impaired PAR2 response, its expression and the metabolic criteria defining metabolic syndrome. In SHRSP.ZF at 30 weeks of age, waist length to body length ratio, blood pressure, serum triglyceride, and serum glucose were 1.3-, 1.7-, 15-, and 1.5-times higher than that of WKY, respectively. As shown in Fig. 3B, in these parameters, only systolic blood pressure correlated with PAR2-mediated vasodilation, but not with PAR2 mRNA expression. Additionally, other any parameters were not correlated with either the vasodilation or the expression.30) In contrast, oxidative stress was identified as a potential mediator. Thiobarbituric acid reactive substances (TBARS) is a serum marker for systemic oxidative stress. We observed 2-fold higher levels of TBARS in SHRSP.ZF that were 20 weeks older than younger animals (10 weeks of age)30); and PAR2 agonist induced vasodilation was inversely correlated with TBARS (Fig. 3B), which is consistent with ROS being inhibitory factors.30) PAR2 mRNA was also inversely correlated with TBARS levels30) (Fig. 3B). In the older animals, we had reported that the responsiveness of arterial smooth muscle to NO is reduced.30) Based on our more recent findings, we propose that increased oxidative stress resulting from chronic exposure to metabolic abnormalities is also an inhibitory driving factor for the age dependent impairment of PAR2 in the vasculature (Fig. 3A).
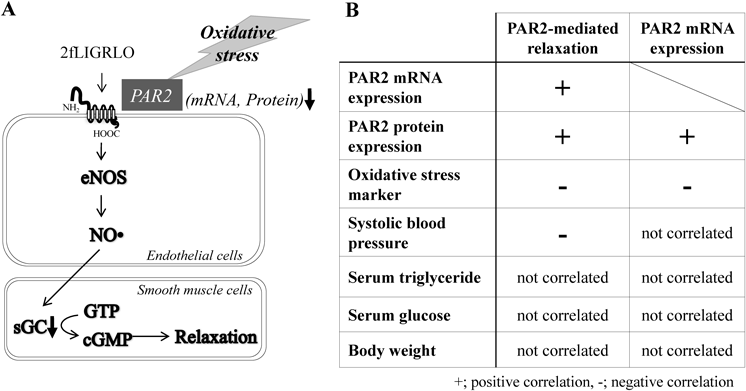
The table are summarized from Maruyama.30) 2fLIGRLO; 2-furoyl-LIGRLO-NH2 (PAR2-activating peptide), NO; nitric oxide, eNOS; endothelial NO synthase, sGC; soluble guanylyl cyclase, cGMP; cyclic GMP, ↓; decrease in mRNA and protein expressions of PAR2.
The observed impairment of PAR2 response in addition to reduction of NO response in arteries may indicate an increased risk of cardiovascular disease at later stage of metabolic syndrome. In patients with metabolic syndrome, the risk for developing cardiovascular disease, e.g., stroke, heart attacks, ischemia, and vasospasm, over a five to ten year period is 1.5- to 3-fold higher than people not having metabolic syndrome.19) Further work is needed to determine whether restoring PAR2 vascular function by treatments that intervene with the inhibitory factors may have potential therapeutic benefit in addition to restoring the ability of the endothelium to regulate vasomotor tone.
PAR2 has multiple physiological effects that could be either favorable or negative for cardiovascular health. To counter inflammation brought about by PAR2 activation, PAR2 antagonists are in development and targeted for clinical applications in treatment of inflammatory diseases.11) PAR2 antagonists (GB-83; GB-88) were protective against arthritis31) and colitis32) in animal studies. GB-88 also reduced adipose inflammation in a diet-induced obesity model.16) PAR2 agonists may have use as vasodilators. There is an accumulated body of evidence indicating that PAR2 activation modulates blood circulation to vital organs, including the heart and brain, and showing protection against damage in disease conditions.33) PAR2 activators have been shown to reduce blood pressure34–36) and be protective against ischemia-injury in multiple organs.37–39) In fact we measured blood pressures in conscious unrestrained by radiotelemetry and found higher systolic blood pressures in mice that lack PAR2 expression.40) Aside from patients with cardiovascular disease and metabolic syndrome, the promise of PAR2-based therapeutics is significant for improvements in human healthy ageing, given that chronic diseases or conditions like inflammation have more impact on the elder population. So, the impact of age and cardiovascular functions on the effectiveness of PAR2 antagonists is timely and warrants further attention.
At early stages of metabolic syndrome, PAR2’s effects on the cardiovascular system are preserved by an increase in endothelial NO production even when NO-mediated responsiveness is attenuated (Fig. 4, left). However, PAR2-mediated vasodilation function is reduced in an age-dependent manner as a result of increased oxidative stress induced by chronic exposing to metabolic abnormalities (Fig. 4, right). The decline of PAR2 function may contribute to the development of cardiovascular dysfunction at later stages of metabolic syndrome. In other words, PAR2 may serve as preservation of blood supply to the organ and blood pressure. In future studies, clarifying the physiological and pathological consequence of the preserved/impaired PAR2-mediated vasodilation in metabolic syndrome should offer more insight into the future of PAR2-based therapeutics to improve healthy ageing.
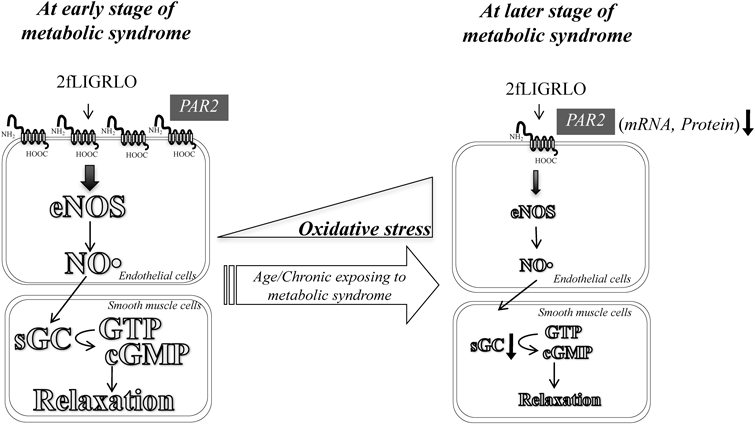
2fLIGRLO; 2-furoyl-LIGRLO-NH2 (PAR2-activating peptide), NO; nitric oxide, eNOS; endothelial NO synthase, sGC; soluble guanylyl cyclase, cGMP; cyclic GMP, ↓; decrease in mRNA and protein expressions of PAR2.
The authors greatly thank Prof. Kazumasa Shinozuka and Dr. Hirokazu Wakuda for their helpful advice. The authors express sincere gratitude to Ms. Yasuko Mino, Ms. Saki Iwata, Ms. Akiko Ogura, and Ms. Natsumi Maruyama for their technical assistance. S. Kagota received a research Grant from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan KAKENHI (Grant number 23590315) for the research highlighted in this review. J. J. McGuire received Grant funding from the Canadian Institutes of Health Research, Canada Foundation for Innovation, Newfoundland Research and Development Corporation for work on PAR2 cited in this review.
The authors declare no conflict of interest.