2017 年 40 巻 4 号 p. 425-434
2017 年 40 巻 4 号 p. 425-434
Gallic acid (3,4,5-trihydroxybenzoic acid, GA), a natural phenolic acid has been reported as a strong antioxidant. Therefore the present study was designed to evaluate the effects of GA and dodecyl gallate (DGA) against acute and chronic carbon tetrachloride (CCl4)-induced hepatotoxicity. For acute model, rats were orally treated with GA and DGA for 7 d prior to CCl4 by intraperitoneally (i.p.) injection. For the chronic model, rats were orally treated with GA or DGA and CCl4 i.p. twice a week for four weeks. In both acute and chronic models, the CCl4-treated groups showed significantly increase in serum hepatic enzyme activities and histopathologic alterations, as well as a disruption in antioxidative status. In contrast, the treatment with GA and DGA restored serum hepatic enzymes activities, improved histopathologic alterations, increased glutathione (GSH) and decreased lipid peroxidation levels. The activities of liver antioxidant enzymes were increased by GA and DGA only in acute model. The expression of p53 gene increased about 3.5 times after GA and DGA treatments, which could result in cell death of damaged hepatocytes preventing of a lifelong liver failure. Thus, these results suggest that GA and DGA has the potential to prevent liver damages as the case of fibrosis condition.
Liver is a vital organ of human body, which performs detoxification of the exogenous xenobiotics, drugs, vital infections and chronic alcoholism. Despite its high regeneration capacity, prolonged liver damage leads to severe inflammation and results in fibrosis, then cirrhosis, and eventually in liver failure.1,2)
Carbon tetrachloride (CCl4) is a potent hepatotoxic chemical, which has been widely used as an experimental animal model of liver injury comparable with human hepatic toxicity involving the aggravation of inflammatory processes and recruitment of inflammatory cells. The toxic mechanism of CCl4 was identified to involve the excessive production of reactive oxygen species (ROS). In the liver, excess of ROS insult may result in liver failure or liver fibrosis.3–5)
Polyphenolic compounds have been suggested as potential therapeutic agents for numerous chronic diseases. Several studies have shown that natural compounds with antioxidant activity are effective in protecting the liver from oxidative damages besides up regulating a group of cytoprotective genes.6–8)
Gallic acid (3,4,5-trihydroxy benzoic acid, GA) is a natural botanical phenolic compound, which is widely distributed in grapes, green tea and pomegrate. Studies have shown that GA processes a lot of biological activities, such as anti-inflammatory, antimutagenic, antioxidant and antitumoral. Gallic acid also induced apoptosis in human cancer cell lines by regulation of mitochondria-dependent pathway, including Bcl2, p53, and Bax. A variety of substituents in the GA acid portion allow the obtainment of esters with a number of analogues with distinct pharmacological properties.9–12)
The difference among the esters derivatives is only in the carbon atom number of the aliphatic side chain, giving them different physicochemical properties, especially lipophilicity. These ester derivatives, especially alkyl esters, demonstrated more favorable pharmacological properties, and in many cases these effects were even stronger than those observed for GA itself. For instance, synthetic GA derivatives with eight or more carbon atoms in the side-chain were more efficient than GA in antiviral, antifungal, antioxidant and anticancer activities, wherein dodecyl gallate (DGA) can be mentioned as the most active.10,11,13,14) This is the first evaluation of GA and DGA as liver injury protector by using both, acute and chronic CCl4 intoxication models. For this purpose, oxidative stress, liver function, lipid profile, histomorphological changes and expression of p53 gene were evaluated.
CCl4, gallic acid (GA) and dodecyl gallate (DGA) were purchased from Sigma Chemical Company, U.S.A. Other chemical reagents were of high-quality analytical grade. CCl4 was diluted with olive oil while GA and DGA solutions were prepared in 0, 1 M phosphate buffer saline pH 7.4 with 1% of dimethyl sulfoxide (DMSO).
Animal CareHealthy adult male Wistar albino rats (180–200 g) were housed in polyethylene cages under controlled laboratory conditions at 22±2°C with a 12 h light/dark cycle maintained with lights on from the time of 07:00 to 19:00, with free access to water and food. They were allowed 1-week acclimatization period before the initiation of the experiment. All procedures were approved by the Institutional Ethics Committee of University of West of Santa Catarina (document number: 004/2014). In all experiments, animals were managed following the principles and guidelines for the care of laboratory animals.
Animals and TreatmentIn brief, the rats were randomly allocated into four or six groups for each model. For acute model (n=6/group), control and CCl4 group orally received 0.1 M phosphate buffer saline pH 7.4 with 1% of DMSO, two groups were orally administered with GA or DGA 100 mg/kg for seven consecutive days. Thirty minutes after the last treatment, CCl4 (1 mL/kg, 50% (v/v) in olive oil) was injected intraperitoneally (i.p.) to all animals except those of the control group.
For the chronic model (n=6/group) the control and CCl4-treated groups orally received 0.1 M phosphate buffer saline pH 7.4 with 1% of DMSO and the others were treated with GA (50, 100 mg/kg) or DGA (50, 100 mg/kg) twice a week for 4 weeks. The CCl4 (3 mL/kg, 30% (v/v) in olive oil) was injected i.p. to all animals, except those of the control group, twice a week, thirty minutes after the treatment with GA or DGA. We chose the maximum dose of 100 mg/kg to avoid hepatic damages according to previous studies.8,15)
In the both models, 24 h after CCl4 injection animals were killed by an overdose of ketamine/xilasine. Blood samples were collected and separated by centrifugation at 800×g for 10 min and the serum samples were biochemicaly analysed. Liver samples were removed for histopathological and biochemical analysis.
Weight Gain (WG)The animals were weighed at the beginning and end of the treatments and the weight gain was determined by the following formula: WG=(final weight−initial weight).
Relative Weight (RW) LiverThe livers were weighed after being immersed in sodium chloride 0.9% for removal the excess of blood and gauze was used to remove excess of liquid from the liver. RW liver, per 100 g of animal was calculated from the following formula: RW=((liver weight÷body weight)×100).
Determination of Biochemical Markers of Hepatic InjurySerum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyltransferase (GGT), albumin and total bilirubin were analyzed according to the manufacturer’s protocol (Labtest Diagnóstica SA, Lagoa Santa, Brazil).
Estimation of Serum Lipid Profile ParametersTriglycerides (TG) and total cholesterol (TC) were assayed by enzymatic colorimetric test using human kits (Labtest Diagnóstica SA, Lagoa Santa, Brazil) according to the manufacturer’s protocol.
Evaluation of Oxidative Stress in LiverLivers were quickly removed and homogenized (liver 1 : 10, w/v) in a buffer containing 1% Triton X-100 150 mM, NaCl 20 mM sodium phosphate, pH 7.4. The livers were homogenized in a tissue homogenizer for 30 s on ice, followed by centrifugation at 10000×g for 10 min. Protein content was determined by Lowry’s method.16) Glutathione peroxidase (GPx) was assayed using 200 µg of protein, and reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidation was monitored spectrophotometrically at 340 nm.17) Catalase activity was determined using 100 µg of protein.18) Glutathione reductase (GR) was assayed using 200 µg of protein and the NADPH oxidation, which resulted from the reduction of oxidized glutathione (GSSG) by GR, determined spectrophotometrically at 340 nm.17,19) Glutathione-S-transferase (GST) was assayed using 100 µg of protein. This assay is based on the conjugation of reduced glutathione (GSH) with 1-chloro-2,4-dinitrobenzene (CDNB) by GST. The conjugate was detected spectrophotometrically at 340 nm.20) Lipid peroxidation was determined by measuring thiobarbituric acid reactive species (TBARS) level, which was determined according to the method already established.21) The concentration of reduced glutathione (GSH) in the samples was determined by the 5,5-dithiobis-2-nitrobenzoic acid (DTNB) method.22) This method is based on the reaction of GSH with DTNB, generating a thiolate anion (TNB), whose yellow color is measured spectrophotometrically at 412 nm.
Histopathological ExaminationLiver sections were embedded in paraffin and sliced into 5 µm thick sections in a rotary microtome and then stained with hematoxylin & eosin dye. The histopathological examination of the slides was performed under a microscope with 40× magnification power. The parameters examined for the assessment of histological damage and their relative score system were as follows according23): i) hepatic architecture: preserved, partial loss or complete loss; ii) hydropic degeneration: absent, mild, moderate or marked; iii) fatty changes: absent or present; iv) central vein congestion: present or absent; v) Kupffer cell hyperplasia: absent or present; vi) necrosis: absent or present; vii) fibrosis: absent or present; vii) infiltration of portal tract by lymphocytes: absent or present.
Quantitative Real-Time RT-PCRTotal RNA was extracted from the liver of animals submitted to chronic model using Trizol (Invitrogen, Carlsbad, CA, U.S.A.) according to the manufacturer’s protocol. Total RNA (2 µg) was used for cDNA synthesis, according to the manufacturer’s instructions for the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, U.S.A.), using random hexamers. This cDNA was subsequently used for quantitative PCR to verify the gene expression levels. Power SYBR-Green PCR Master Mix (Applied Biosystems) was used to employ quantitative PCR. The sequences of specific primers used for each gene studied, including the endogenous control GAPDH, are shown in Table 1. Briefly, a reaction contained SYBR Green PCR Master Mix, forward and reverse primers (final concentration 0.15 µM) and the template cDNA. The PCR was performed using ABI 7900 at 50°C for 2 min, at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, and 57°C for 1 min. Nonspecific amplification and primer/dimer artifacts were verified through a dissociation curve reaction immediately after the completion of the real-time PCR run. The gene-specific product was normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and quantified using the comparative (ΔCt) Ct method as previously described.24)
| Gene | Foward primer 5′–3′ | Reverse primer 5′–3′ | Product size (bp) |
|---|---|---|---|
| p53 | GTAAACGCTTCGAGATGTCC | GACTGGCCCTTCTTGGTCT | 123 |
| GAPDH | GTGTCCGTCGTGGATCTGAC | GGAGACAACCTGGTCCTCAG | 132 |
All data were expressed as the mean±standard error of mean (S.E.M.). For statistical evaluation, ANOVA with the Tukey’s test or Dunnett’s test were used to compare all groups or to compare with the control group, respectively. p<0.05 was considered a statistically significant difference.
The acute carbon tetrachloride intoxication was able to increase the relative liver weight and triglycerides levels compared with the non-treated rats. The pretreatment with DGA (100 mg/kg) during seven days significantly prevented (p<0.05) the increase of relative liver weight and levels of TG (Table 2).
| Treatment | Weight gain (g) | Relative liver weight (g) | TG (mg/dL) | TC (mg/dL) |
|---|---|---|---|---|
| Control | 31.4±5.7 | 3.31±0.16 | 60±4.2 | 105±15.0 |
| CCl4 | 30.6±2.6 | 4.65±0.30* | 98±8.9* | 92±6.1 |
| GA 100 mg/kg+CCl4 | 38.6±2.4 | 4.24±0.14* | 86±7.2 | 94±6.4 |
| DGA 100 mg/kg+CCl4 | 26.2±5.1# | 3.96±0.15 | 65±8.3# | 88±7.3 |
TG, triglycerides; TC, total cholesterol. Mean±S.E.M. (n=6 number). * Significance from the control group at p<0.05 probability level, using ANOVA and Dunnet’s test. # Significance from the CCl4 group at p<0.05 probability level using ANOVA and Tukey’s test.
In acute model, the total bilirubin, ALT, AST and GGT levels of the CCl4 alone treated group were markedly increased when contrasted with the control group (Table 3). The marked protective effects of GA or DGA can be observed on serum markers of liver injury, compared to the group of CCl4 alone (p<0.05).
| Treatment | Albumin (g/dL) | Total bilirubin (mg/dL) | ALT (U/L) | AST (U/L) | GGT (U/L) |
|---|---|---|---|---|---|
| Control | 2.9±0.15 | 0.34±0.02 | 48.7±4.0 | 104±2.3 | 37.5±0.5 |
| CCl4 | 2.7±0.04 | 0.8±0.05* | 335.2±28.5* | 426.2±72.0* | 171.2±19.0* |
| GA 100 mg/kg+CCl4 | 2.8±0.07 | 0.5±0.06# | 202.7±65.0*,# | 289±86.6*,# | 142±20.0* |
| DGA 100 mg/kg+CCl4 | 2.7±0.11 | 0.58±0.07# | 195±13.7*,# | 293±46.0*,# | 47.2±5.3# |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, γ-glutamyltransferase. Mean±S.E.M. (n=6 number). * Significance from the control group at p<0.05 probability level, using ANOVA and Dunnet’s test. # Significance from the CCl4 group at p<0.05 probability level using ANOVA and Tukey’s test.
Treatment with CCl4 in acute model showed an increase in TBARS levels with decrease in GSH levels, whereas Catalase, GPx, GR, GST activities decreased when contrasted with the control group (Fig. 1). Gallic acid and DGA 100 mg/kg were able to restore the antioxidant status with increase in GSH levels and decrease in TBARS (Figs. 1A, B). Even so, it was observed an increase in catalase and GPx activity with GA treatment while treatment with DGA improved GPx, GR and GST activities, when compared to CCl4-treated group (Figs. 1C–E).
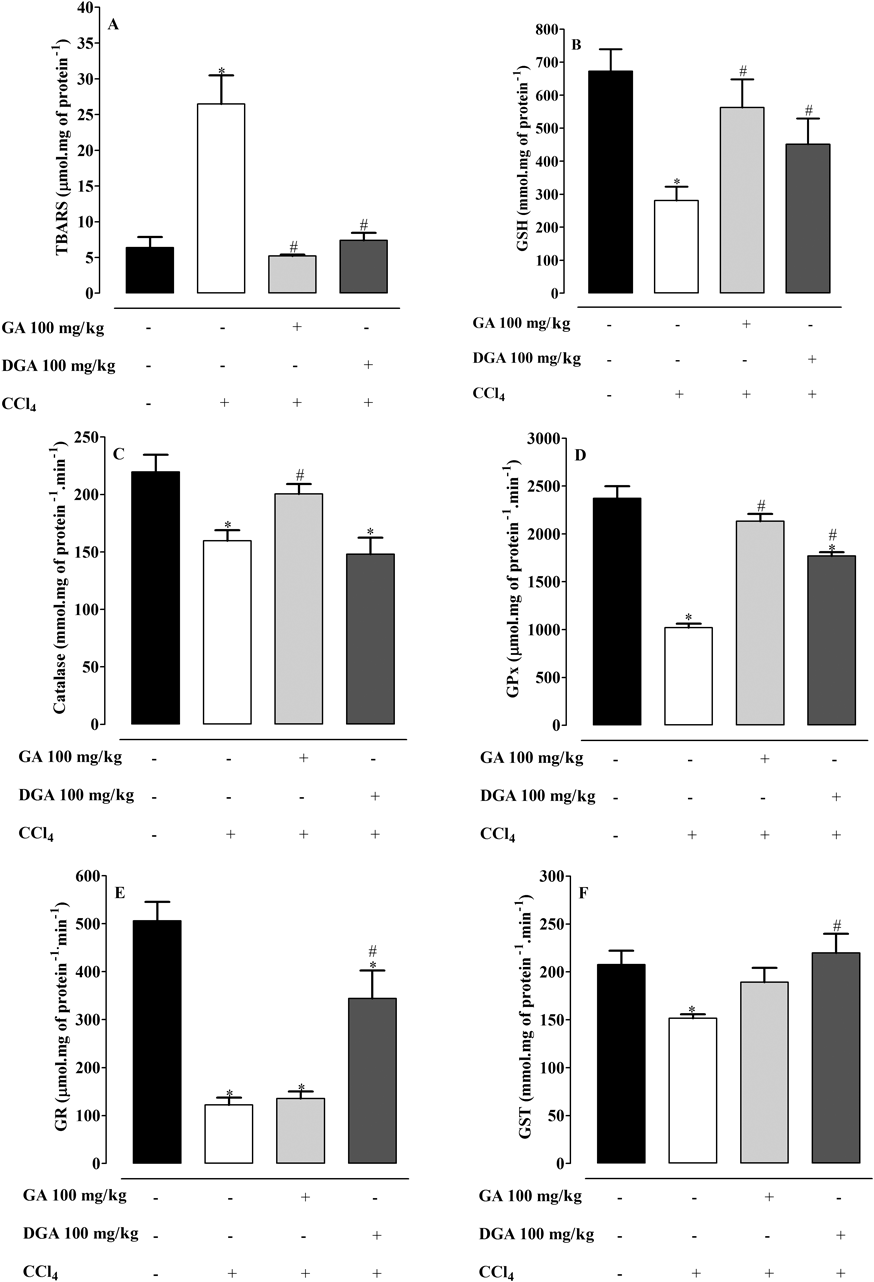
Mean±S.E.M. (n=6 number). * Significance from the control group at p<0.05 probability level, using ANVOA and Dunnet’s test. # Significance from the CCl4 group at p<0.05 probability level using ANOVA and Tukey’s test.
Hematoxylin & eosin stain for sections of normal control group showed structural integrity without necrosis, inflammation, or fibrosis development (Fig. 2A). In contrast, in acute model, CCl4 induced severe loss of the hepatic architecture, marked hydropic degeneration, fatty changes, central vein congestion, and necrosis medium lobular with intense vacuolated hepatocytes (Fig. 2B). The pathological changes induced by CCl4 were substantially improved in the groups treated with GA or DGA 100 mg/kg. The architecture was preserved in 30 and 60% of animals treated with 100 mg/kg of GA or DGA, respectively. The treatment with DGA 100 mg/kg decreased necrosis score as compared with the CCl4-treated group.
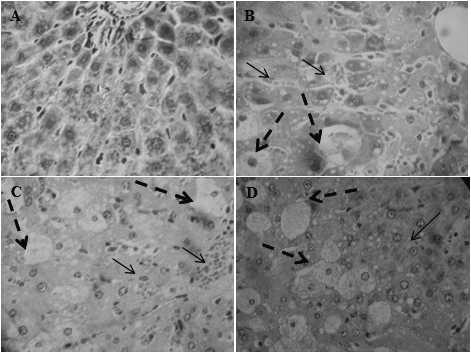
Histological changes are shown bonnets staining hematoxylin & eosin (H&E 40×). (A) Representative image of the control group liver. (B) Representative image of the group treated with CCl4 (0.5 mL/kg). (C) Representative images of the group treated with GA 100 mg/kg 7 d and co-treated with CCl4 0.5 mL/kg on day 7. (D) Representative images of the group treated with DGA 100 mg/kg 7 d and co-treated with CCl4 0.5 mL/kg on the 7th day. Continuous arrows coagulative necrosis; segmented arrows vacuolated hepatocytes.
Since treatment with GA and DGA in acute liver toxicity caused by CCl4 showed significant liver protection, we decided to test them in a chronic model of hepatotoxicity induced by CCl4, in which occurs the development of liver fibrosis. The same parameters were evaluated in the chronic model at doses of 50 and 100 mg/kg, adding to study the expression of the p53 gene, since changes in this gene may be associated with increased cellular degeneration.
The p53 protein is involved in various biological functions including cell cycle arrest, cellular senescence and apoptosis.25) It is able to transcriptionally regulate various pro-apoptotic genes leading to cell death.26) Many studies have reported links between p53 and ROS. Evidences have shown that p53 possesses potent redox-regulating activity through regulating various ROS-generating and antioxidant enzymes.27–29) Increasing cellular ROS promotes p53 expression which induces pro-oxidant genes resulting in cell death. However, other studies report that p53 is associated with the expression of antioxidant genes such as GPx and SOD, showing that p53 also has a protective function and is involved in antioxidant defense system. Therefore we thought that the evaluation of this gene could help in the understanding of the action of gallic acid and dodecyl gallate against a stressing agent like CCl4.
Effect of Gallic Acid and Dodecyl Gallate against Chronic Carbon Tetrachloride-Induced HepatoxicityThe stress oxidative promoted for administration of CCl4 have play a key role in the body weight and the organ weight of rats. Chronic carbon tetrachloride intoxication caused significant reduction (p<0.05) in body weight and an increase in tissue weight as compared with the control group. The treatment with GA or DGA of 50 and 100 mg/kg body weight significantly restored (p<0.05) the weight of body as well as the relative liver weight (Table 4). The alterations in the levels of serum lipids in normal and experimental rats are presented in Table 4. Treatment with CCl4 significantly increased lipid profile (triglycerides and total cholesterol); however this effect was reversed by the treatment with GA or DGA at 50 and 100 mg/kg body weight, respectively.
| Treatment | Weight gain (g) | Relative liver weight (g) | TG (mg/dL) | TC (mg/dL) |
|---|---|---|---|---|
| Control | 42.8±2.82 | 3.94±0.11 | 66±1.9 | 90±3.9 |
| CCl4 | −3.3±0.88* | 5.02±0.14* | 141±7.7* | 125±5.2* |
| GA 50 mg/kg+CCl4 | 26.5±0.99*,# | 4.25±0.07# | 92±9.4# | 85±5.6# |
| GA 100 mg/kg+CCl4 | 27.0±1.46*,# | 4.33±0.04# | 91±11.0# | 82±9.7# |
| DGA 50 mg/kg+CCl4 | 20.5±1.38*,# | 4.41±0.07# | 88±5.3# | 84±7.2# |
| DGA 100 mg/kg+CCl4 | 5.5±1.33*,# | 4.96±0.19* | 97±12.0# | 92±4.9# |
TG, triglycerides; TC, total cholesterol. Mean±S.E.M. (n=6 number). * Significance from the control group at p<0.05 probability level, using Dunnet’s test. # Significance from the CCl4 group at p<0.05 probability level using Tukey’s test.
In chronic model, the total bilirubin, ALT, AST and GGT levels of the CCl4 alone treated group were markedly increased when contrasted with the control group (Table 5). The marked protective effects of GA or DGA can be observed on serum markers of liver injury when compared to the group of CCl4 alone (p<0.05).
| Treatment | Albumin (g/dL) | Total bilirubin (mg/dL) | ALT (U/L) | AST (U/L) | GGT (U/L) |
|---|---|---|---|---|---|
| Control | 2.8±0.06 | 0.31±0.03 | 57.6±3.9 | 113±2.3 | 52±5.5 |
| CCl4 | 1.8±0.13 | 1.4±0.11* | 524±30.0* | 645.2±16.0* | 261.8±25.0* |
| GA 50 mg/kg+CCl4 | 2.3±0.12 | 0.51±0.08*,# | 138.2±25.0*,# | 147±4.4*,# | 99±20.2*,# |
| GA 100 mg/kg+CCl4 | 2.9±0.10# | 0.87±0.15*,# | 82.5±12.4*,# | 62±3.5*,# | 95±11.6*,# |
| DGA 50 mg/kg+CCl4 | 2.9±0.03# | 0.48±0.06*,# | 99±13.4*,# | 98±13.5*,# | 164.7±13.0*,# |
| DGA 100 mg/kg+CCl4 | 2.5±0.22# | 0.91±0.13*,# | 154.7±65.0*,# | 175.2±14.0*,# | 161±5.3*,# |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, γ-glutamyltransferase. Mean±S.E.M. (n=6 number). * Significance from the control group at p<0.05 probability level, using Dunnet’s test. # Significance from the CCl4 group at p<0.05 probability level using Tukey’s test.
Treatment with CCl4 in chronic model showed an increase in TBARS levels with decrease in GSH levels, whereas Catalase, GPx, GR, GST activities decreased when contrasted with the control group (Fig. 3). Treatments with GA or DGA induced a high decrease in TBARS levels in about 50% and an increase in GSH levels in about 400% when compared with CCl4-treated group (Figs. 3A, B). The antioxidant status was not greatly improved in groups treated with GA or DGA (Figs. 3C–F). The only alteration observed was an increase in GR activity induced by GA 50 mg/kg (Fig. 3E) when compared with CCl4 group.
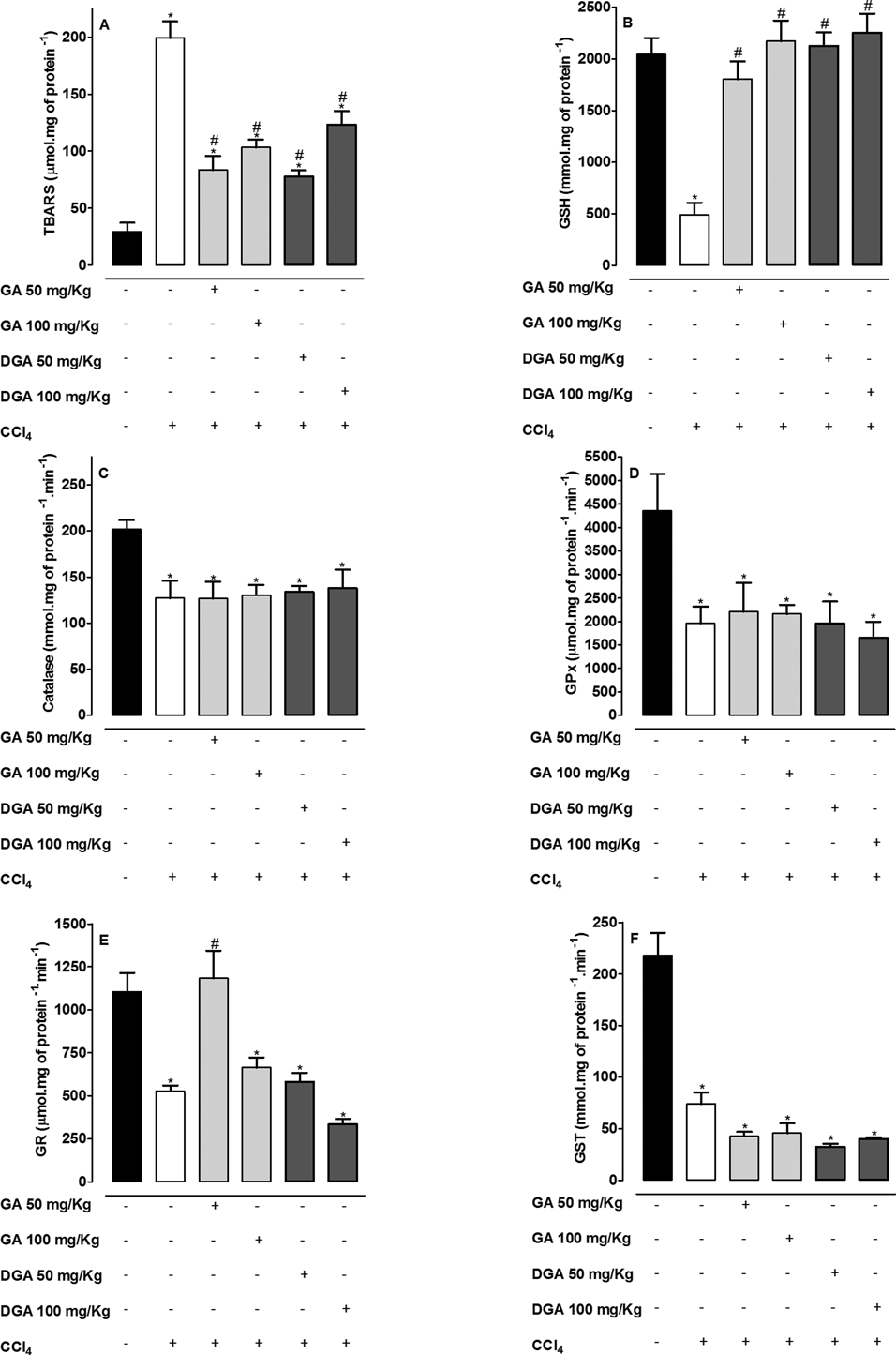
Mean±S.E.M. (n=6 number). * Significance from the control group at p<0.05 probability level, using ANOVA and Dunnet’s test. # Significance from the CCl4 group at p<0.05 probability level using ANOVA and Tukey’s test.
Histopathological observations of liver sections from the control group showed normal cellular architecture with distinct hepatic cells, sinusoidal spaces (Fig. 4A). In contrast, the CCl4 group exhibited the most severe damage of any of the groups. The liver sections in this group 100% of rats showed severe hepatic fibrosis and necrosis, 100% fatty degeneration and moderate bile duct hyperplasia, with addition of discrete megalocytosis in 80% and mitosis in 40% of rats (Fig. 4B). The liver sections of the rats treated with GA 50 mg/kg (Fig. 4C) only 30% of them showed a discrete fibrosis and megalocytosis, 30% fatty degeneration and 60% mitosis. The treatment with GA 100 mg/kg (Fig. 4D) only 10% of rats showed fibrosis without megalocytosis, 30% fatty degeneration and 60% mitosis. Treatment with DGA 50 mg/kg showed a slight fibrosis (20% of rats) with 80% the presence of moderate fatty degeneration without megalocytosis and 80% of rats showed mitosis (Fig. 4E). DGA 100 mg/kg decrease fatty degeneration (60% of rats), showed 20% of fibrosis and 100% of mitosis without megalocytosis (Fig. 4F).
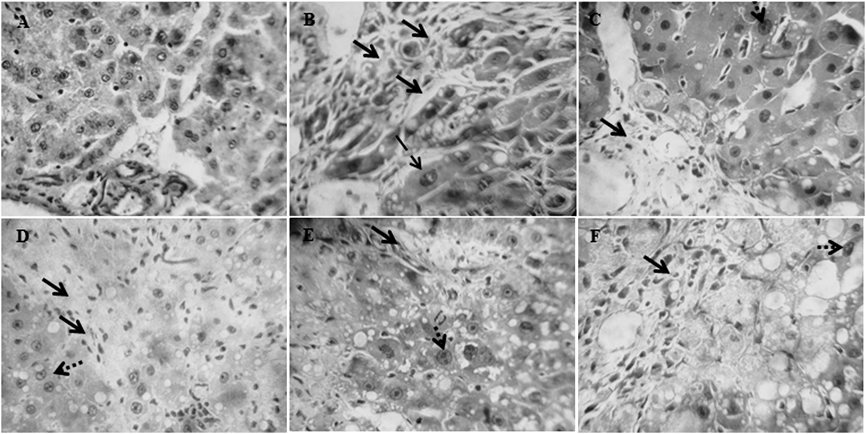
The animals were exposed to CCl4 twice a week for 4 weeks and on the same days treated with GA (50 or 100 mg/kg) or with DGA (50 or 100 mg/kg). Histological changes are shown after staining with hematoxylin & eosin (H&E 40×). (A) Representative image of control group (no change). (B) Representative image of the CCl4-treated group. (C) Representative image of the group treated with GA 50 mg/kg. (D) Representative image of the group treated with GA 100 mg/kg. (E) Representative image of the group treated with DGA 50 mg/kg. (F) Representative image of the group treated with DGA 100 mg/kg. → hepatic fibrosis; – → megalocytosis; 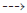 mitosis.
mitosis.
Analyses on p53 expression are showed in Fig. 5 and demonstrate that GA and DGA in all concentrations induced a strong increase in p53 gene expression when compared with the CCl4 group. There was a difference between the dose of 50 and 100 mg/kg, therefore the effect does not appear dose-dependent. In a pilot study, gallic acid and dodecyl gallate did not increase expression of the p53 gene when given alone at a dose of 100 mg/kg for 1 week (results not shown).
Real time RT-PCR analyses were performed by real time RT-PCR in liver tissues after chronic treatment with CCl4 and co-treatment with gallic acid (GA) or dodecyl gallate (DGA) at 50 and 100 mg/kg. GAPDH was used as endogenous control and the expression level of genes in untreated group was designated as 100%. Mean±S.E.M. (n=6 number). * Significance from the control group at p<0.05 probability level, using ANOVA and Dunnet’s test. # Significance from the CCl4 group at p<0.05 probability level using ANOVA and Tukey’s test.
The liver injury induced by CCl4 have been reported for a large number of animal model.8,15,23,30–32) The metabolic process of CCl4 generates free radicals, which covalently bind to the cell proteins and increases lipid peroxidation and membrane disintegration, with eventually cell fibrosis and necrosis.6,19,33) Damages to the parenchymal and non-parenchymal liver cells result in elevations of the both ALT, AST and GGT, which have been widely accepted as major biomarkers to assess the hepatic injury.2,34,35) The increase in serum level of these markers indicates a loss of liver cell membrane integrity, which might be directly related to an increase in ROS CCl4-induced.36,37)
In our study, we observed an obvious increase in the levels of TG about 63 and 113% in acute and chronic model respectively and TC about 38% in chronic model, which is in accordance with previous reports.19,38) Lipids are a heterogeneous group containing active metabolic substances that play an important role in the pathogenesis of liver disease. The most common lipid abnormalities in liver damage caused by CCl4 is hypercholesterolemia and hypertriglyceridemia.39)
Increased TG levels after ethanol or CCl4 ingestion may be due to the increased availability of free fatty acid (FFA), glycerophosphates, decreased TG lipase activity, and decreased fatty oxidation. These increased TG levels may lead to increased availability of FFA for esterification. Free fatty acids are the principal components present in most lipids of biological importance. Reports show that an increase in FFA level can increase the synthesis of other major lipids and activate NADPH- or NADH-dependent microsomal peroxidation.40,41) Probably CCl4 ingestion may produce the formation of esterification products of fatty acids, which may be the mediators of end-organ damage.
With the above-mentioned observation we found that treatment with GA and DGA in acute and chronic intoxication restored the lipid levels to near normal indicating that these compounds could have antihyperlipidemic activity. The hypolipidemic effects of GA was already reported,42) but for DGA it was never reported and its effect was even higher reducing about 60% of TG levels.
Our results clearly show that the CCl4 administration induced liver injury with changes in serum transaminases (ALT and AST) and GGT, total bilirubin and albumin. These changes are related to the capacity of CCl4 induce oxidative stress also evidenced by the levels of TBARS and GSH.
Our data suggest that GA and DGA are able to combat toxic radicals, since in both acute and chronic models, they inhibited or modulated serum-stimulated events and prevented liver damages as observed in histopathologic analysis. In acute and chronic models, GA and DGA treatments significantly reduced necrotic and fibrotic areas, respectively, which could be related to inactivation or prevention of the excessive ROS production. These data are in accordance with those found by Wang et al.,43) which observed that GA (15 mg/kg) reduced fibrotic area, inflammatory infiltration, steatosis and levels of malondialdehyde (MDA), ALT, AST and GGT when compared CCl4-injured group.
Another enzyme frequently altered in liver damages is GGT, which catalyzes the first step of GSH degradation.44) Increases in serum GGT as observed in CCl4 treated group means an overproduction of free radicals.23) The treatment with GA and DGA was able to reduce significantly GGT, others serum enzymes activities and total bilirubin when compared with CCl4 group.
To further confirm the hepatoprotective effect of GA and DGA, the plasma albumin concentration in CCl4-intoxicated rats was measured. Decreased plasma albumin levels indicate chronic liver failure caused mainly by cirrhosis, a late stage of hepatic fibrosis when there is a widespread distortion of the normal hepatic architecture.19) In the chronic model a decrease in plasma albumin levels was observed after CCl4 intoxication, effect recovered after GA and DGA treatments.
Several studies in vitro and in vivo made in our laboratory show that the derivatives of gallic acid with more than 8 carbons in the side chain do not significantly alter the levels of hepatic and renal biomarkers (albumin, ALT, AST, GGT, urea and creatinine).10,13,45–47) The results of this study suggest that GA and DGA present a hepatoprotective effect against the toxic action promoted by CCl4. The results of Table 5 indicate that the effect of GA seems to be dose dependent, while the effect of the DGA is dose-independent. These different effects may be associated with the differing levels of hydrophobicity associated with each compound. Studies carried out by Dodo et al.,48) Rosso et al.,14) and Locatelli et al.,45) indicated that the hydrophobicity of the alkyl chain of gallate derivatives is important in terms of antiproliferative and antioxidant activity, and the alkyl chain might contribute to improving the cell permeability or the interaction with hydrophobic pockets in the target molecule.
Other interestingly result obtained from the chronic model was the significant reduction in body weight and an increase in liver weight in CCl4-intoxicated rats. Conversely, the treatments with GA (50, 100 mg/kg) and DGA (50 mg/kg) were able to avoid the loss of weight. This effect could be related to the reduction in inflammation and in liver damages, as observed in histological data. Others works have showed that gallic acid could suppress the release of pro-inflammatory cytokine interleukin (IL)-6, IL-31- and IL-33 and pretreatment with GA ameliorates myocardial injury induced by nano-alumina, probably through its hypolipidemic, anti-inflammatory, and antioxidant effects.49,50)
Previous studies using rutin (50, 70 mg/kg) in the treatment of hepatotoxicity CCl4-induced showed similar results, with a significant reduction in the amounts of serum enzymes ALT, AST and GGT hepatic, lipid peroxidation and an increase in liver GSH.13) Tirkey et al.51) showed similar results using hesperidin at doses of 100 and 200 mg/kg.
Treatment with CCl4 in both models showed a significant reduction in the activity of antioxidant enzymes CAT, SOD, GPx, GR and GST and these results are in agreement with others related to such kind of liver damages.11,43,49) Liver contains high endogenous expression of antioxidant enzymes, such as SOD and GPx, which are the primary defense mechanisms against ROS. SOD removes superoxide and protects cells from oxidative damages, and GPx protects the structural integrity of cell membranes.33,38) Surprisingly we found that the treatments with GA and DGA were unable to increase the activity of the antioxidant enzymes as SOD, GPx, catalase and GST despite of the GSH, TBARS and serum markers have been changed. These results are probably related with the increase in approximately 50% in GSH levels in the acute model and 400% in the chronic model, as well as by the increased expression of the p53 gene.
On the other hand, in previous studies carried out in our laboratory we observed that the gallic acid derivatives with more than 8 carbons are not able to alter the activity of the antioxidant enzymes CAT, GPx, GR, SOD and GST when administered alone. However, they show changes in these markers when given in animals with tumors or in tumor cells.10,13,45–47) These results lead us to believe that gallic acid and its derivatives selectively act on damaged cells and tissues.
It is widely known that the GSH is an intracellular antioxidant, which participates in detoxification and antioxidant defense. Recently, it has been related high GSH levels with the γ-glutamyl-cysteine synthase (γ-GCS) activity increase. The enzyme γ-GCS participates in de novo GSH synthesis, which involves a rate-limiting step.52) Yang et al.53) showed that GSH reestablishment is followed by the recovered cellular redox status with decreased oxidative stress in γ-GCS overexpressed cells.
The p53 protein is involved in various biological functions including cell cycle arrest, cellular senescence and apoptosis.25) It is able to transcriptionally regulates various pro-apoptotic genes leading to cell death.26) Furthermore, studies in animal p53-null and wild-type (WT) showed that p53 in the liver is a limiting factor between the regeneration and tumor development. Animals p53-null when exposed to hepatotoxic agents show a progression in tumor development, while in WT p53 animals was observed an increase in mitotic and liver regeneration.54,55)
Previous studies showed that CCl4 caused marked reduction in p53. This result may be explained on the basis that CCl4 acts as a tumor promoter through increasing the intracellular concentration of ROS necrosis/regeneration and cell proliferation and/or may be due to p53 mutation. The treatment of CCl4-intoxicated rats with rutin was able to restore the p53 and markedly reduce the DNA damage.5) In the present study, we observed a non-significant reduction in p53 expression in CCl4-intoxicated animals but a strong increase in p53 expression in GA or DGA-treated animals. This effect could be related to a protective factor in liver as previous demonstrated by other substances.54,55) Our findings suggest that the increase in p53 expression promoted by GA and DGA could be favoring cellular rehabilitation instead of elimination them after DNA damage.
These findings suggest that GA and DGA positively modulate the nonenzymatic antioxidant status and regenerate the liver to near normal status. The increase in expression p53 gene may be associated with modulation of antioxidant status, since an increase in hepatic GSH levels show relation with liver regeneration of animals treated with GA and DGA. When the animals were treated with GA and DGA was observed a restoration of hepatic function with decreased area with apoptosis, necrosis and fibrosis.
The increased p53 gene can lead to selective apoptosis, only in damaged cells, since histopathological analysis showed cell death with an increase in mitosis in GA and DGA-treated groups. The presence of mitosis suggests that GA and DGA can support the liver regeneration possibly aiding in the treatment of liver diseases associated with oxidative stress. Our results corroborate with several studies showing that GA and its esters are capable of promoting apoptosis increasing caspase 3 and Bax in tumor cells without causing significant damage to normal cells.13,46) Also, they presented antioxidant activity by inhibiting ROS production as well as scavenging them.14,56) In this way we understand that the increase in p53 gene expression induced by gallic acid and dodecyl gallate is related to the antioxidant status and not to the apoptotic events, since these compounds present high antioxidant power.
Another studies in liver tissue report that p53 is associated with the expression of antioxidant genes such as the GPx and SOD, showing that p53 also has a protective function and is involved in antioxidant defense system.57) Studies by Hussain et al.28,58–60) show that increase in cellular ROS content promote deletion in the p53 gene, increasing the expression of proteins such as Bax favoring the process of apoptosis. On the other hand, the increase in p53 gene expression is associated with the increase GSH content hepatic, this leads to the reduction of the lipid peroxidation and restoration of the cellular function. Our results corroborate with these studies because we observed a significant increase in p53 gene with a reduction in the activity of antioxidant enzymes GPx, GST and CAT and significant increase in hepatic GSH content. Yang et al.53) shown that betulinic acid in hepatocellular carcinoma promoted increase of expression p53 protein and decrease SOD2 this result in antitumoral effect.
In conclusion, we demonstrated that GA and DGA have several benefits in CCl4-intoxicated rats, including: 1) reducing plasma AST, ALT and GGT levels; 2) reducing the increase in liver weight and body weight loss; 3) and reducing lipid peroxidation, necrosis and fibrosis in liver; 4) improve nonenzymatic antioxidant status; 5) and increase expression of p53 gene which seems to be an important factor for liver cells regeneration; 6) increase in expression of p53 gene, which seems to favor apoptosis of damaged cells. Further studies would be complementary to elucidate the mechanisms of action and explore its medicinal value in liver disorders.
The studies developed by our group were supported by Grants from CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), which provided a scientific initiation for Camila Katerin Perondi and by University of West of Santa Catarina, Campus Videira. The authors are also grateful to Universidade do Estado de Santa Catarina for the histological analyses.
The authors declare no conflict of interest.