2017 Volume 40 Issue 6 Pages 758-764
2017 Volume 40 Issue 6 Pages 758-764
The effects of different combinations of ciprofloxacin (CIP) and norfloxacin (NOR) against Escherichia coli and Staphylococcus aureus were studied using checkerboard, fractional inhibitory concentration (FIC) and time–kill analysis methods. Results obtained by the checkerboard method showed that the more effectives combinations against Escherichia coli were 0.0009 µg/mL CIP+0.0312 µg/mL NOR and 0.0037 µg/mL CIP+0.0075 µg/mL NOR with a FIC index of 0.62. For Staphylococcus aureus, the combination of 0.0625 µg/mL CIP+0.2500 µg/mL NOR showed a synergistic effect, with a FIC index of 0.50. The results of the time–kill method demonstrated either indifference or additivity of the combinations 0.0009 µg/mL CIP+0.0312 µg/mL NOR, 0.0018 µg/mL CIP+0.0312 µg/mL NOR, 0.0037 µg/mL CIP+0.0075 µg/mL NOR and 0.0037 µg/mL CIP+0.0156 µg/mL NOR at 24 h against E. coli. The combination 0.0037 µg/mL CIP+0.0312 µg/mL NOR showed synergistic activity. All the analyzed combinations evidenced bactericidal effects at 4 h. The combinations 0.0625 µg/mL CIP+0.2500 µg/mL NOR and 0.0625 µg/mL CIP+0.0625 µg/mL NOR showed indifference or additivity against S. aureus. None of them generated bactericidal effect at 4 h. Moreover, this last equimolecular combination (equivalent to 1/4 minimum inhibitory concentration (MIC) CIP+1/16 MIC NOR) generated higher reduction of nitro blue tetrazolium than drugs alone. By another way, combinations not equimolecular of CIP and NOR assayed, generated less levels of reactive oxygen species (ROS) than the components alone.
Combination therapies are employed to improve antibacterial activity, to reduce the amount of each antibiotic used and the toxicity of drugs, to obtain synergistic antimicrobial activity and to reduce the risk of antibiotic resistance arising during the therapy.1)
Fluoroquinolones (FQs) are a pharmacochemical class of antibacterials widely used as anti-infective agents in clinical medicine. Their potent activity against Gram-positive, Gram-negative, anaerobic and mycobacteria species, coupled with their good pharmacokinetic properties are the main reasons for their therapeutic importance. However, resistance is rising among some organisms.2) Nowadays, not only is discovery of new analogs and new dosage forms in continuous progress, but the study of FQs in combination with several families of antibiotics in vitro is frequent.2–6) FQs exert their antibacterial action by interfering with the function of two essential bacterial enzymes, DNA gyrase and topoisomerase IV, involved in the DNA synthesis.7) Although some overlap may exist, FQs of first, second and third generation exhibit a pattern of specificity with respect to those enzymes. It was thought that gyrase was the primary target for quinolones in Gram-negative bacteria, and that topoisomerase IV was the primary cellular target in Gram-positive strains. Afterwards, new data suggested that fourth-generation quinolones have a dual-binding mechanism of action, inhibiting both DNA gyrase and topoisomerase IV, in Gram-positive species.8–12) Ultimately, the issue is still a matter of debate, and the relative contributions of gyrase versus topoisomerase IV to quinolone action need to be evaluated on a species-by-species and drug-by-drug basis.13)
The exact nature and the molecular organization of the enzyme–quinolone–DNA complex are not known, and different hypotheses have arisen regarding it.14–18) None of the models described is completely satisfactory, so new experimental and theoretical contributions will be of value to explain their molecular mechanism of action. Some of the existing theories propose the formation of dimers and tetramers of FQ molecules, that of Shen et al. being the first and still one of the most widely accepted theories.
As part of our ongoing interdisciplinary chemistry project, we previously reported the preparation and physicochemical characterization of a unique co-crystal, referred to as COP, containing zwitterions of ciprofloxacin (CIP) and norfloxacin (NOR).19) In this co-crystal phase there are three independent drug molecule sites which accommodate different proportions of CIP and NOR such that the overall stoichiometry is 1 : 1. In addition to the presence of strong charge-assisted N+–H ... −O–C hydrogen bonds that link the zwitterions into infinite chains in this co-crystal, two other prominent intermolecular interactions that contribute to crystal stabilization are present, namely strong π–π stacking of the FQs rings and tail-to-tail hydrophobic association of the alkyl moieties (N-cyclopropyl and N-ethyl for CIP and NOR, respectively). Interactions of the latter types are indeed precisely those invoked by Shen et al. in their cooperative binding model for the inhibition of DNA gyrase by quinolone antibacterials.14) At the time that this model was proposed, the crystal structure of the bioactive quinolone nalidixic acid, which features stacking of the naphthyridine rings and tail-to-tail hydrophobic interactions between the N-ethyl groups, was cited as support for their hypothesis, but the same types of intermolecular interactions are evident in crystal structures of FQs antibiotics investigated in more recent years (e.g. those of COP and a triclinic modification of CIP).19–21) Figure 1 highlights the above molecular interactions as they occur in the COP co-crystal phase.

Isolated spheres represent oxygen atoms of water molecules.
Our results with the co-crystal COP prompted us to consider it of interest to evaluate the feasibility of a similar behaviour of CIP and NOR on their antibacterial effect. As far as we know, there are no previous reports concerning the antimicrobial activity of FQ combinations. This paper describes the results of such in vitro exploration and highlights the peculiar propensity of NOR and CIP molecules to act associatively. Determinations of susceptibility of Staphylococcus aureus and Escherichia coli to combinations of CIP and NOR were performed by Fractional Inhibitory Concentration (FIC) and time–kill curve techniques.
On the other hand, recent investigations indicated that CIP can stimulate the generation of reactive oxygen species (ROS) in bacteria,22,23) contributing significantly to bacterial cell death.24) For this reason, it was also of interest to explore the influence of the mixture of CIP and NOR on the production of ROS in S. aureus.
It is worth mentioning that a finding of synergism or indifference in the antibacterial action of combinations of CIP and NOR, would not only impact on their pharmacodynamic profile, but also on their biopharmaceutics properties. According to the Biopharmaceutics Classification System, NOR is classified as a Class IV drug, and CIP falls between Classes II and III.25) This means that NOR presents very low aqueous solubility and poor permeability and that CIP is very soluble at pH below 6 but the solubility decreases at higher pH, and it has poor permeability. In this way, the problem of the low solubility could be overcome if the FQs were more active in combinations where each of them is present in lower concentration.
Finally, at least three mechanism of resistance to FQs are described and it is clear that the relative involvement of them depends on the structure of each drug, among other factors.26,27) On the other hand, differences on the safety profile of FQs are also attributable to the structure of FQs.28–30)
To gain a first insight into the activity of FQ combinations against Gram-negative and Gram-positive bacteria, we used in this work reference strains of Escherichia coli ATCC 25922 and Staphylococcus aureus ATCC 29213, as recommended by the American Society for Microbiology.31)
Antimicrobial agents were supplied by their manufacturers as laboratory standard powders, including CIP from Roux Ocefa S.A. (Buenos Aires, Argentina) and NOR from Finadiet (Buenos Aires, Argentina).
For antimicrobial activity determinations of each FQ against a selected reference organism, minimum inhibitory concentration (MIC) values of CIP and NOR were determined by using the macrodilution method with Mueller–Hinton broth. MICs were measured in triplicate and determined according to standardized methods.31) Stock solutions were prepared immediately prior to testing. FQs were dissolved in 0.5 M NaOH (0.04 mg/mL) before being diluted to appropriate concentrations in sterile distilled water. Serial two-fold dilutions of antimicrobial agents were made. Final antibiotic concentration ranged from 0.0156 to 4.0000 µg/mL (CIP: 4.70×10−5 to 1.20×10−2 µM and NOR: 4.89×10−5 to 1.25×10−2 µM) for S. aureus and from 0.0019 to 0.2500 µg/mL (CIP: 5.72×10−6 to 7.53×10−4 µM and NOR: 5.96×10−6 to 7.83×10−4 µM) for E. coli, in accordance with the sensitivity reported for each strain.5,32–35) Suspensions of 0.5 McFarland standard of organisms were prepared in 0.9% sterile saline from a stationary phase inoculum of bacterial growth on a Mueller–Hinton plate. This was diluted such that an inoculum of approximately 5.0×105 colony forming units (CFU)/mL was obtained. After 20 h incubation at 35°C, the presence or absence of growth was observed for each tube. The MIC was defined as the lowest concentration of antibiotic that inhibited all visible growth.
The checkerboard test complemented with time–kill curve determinations were carried out to study the antibacterial effect of the combination of CIP and NOR. They first were assessed by checkerboard tests, in replicates of six, against each microorganism. Serial dilutions of two different antimicrobial agents were mixed in Mueller–Hinton broth. The concentration range of each antimicrobial agent in 49 combinations ranged from 1/32 times the MIC (1/32×MIC) to 2×MIC, in order to observe the occurrence and magnitude of the synergism or antagonism, as recommended by the standardized methods.31) This corresponds to concentrations of CIP between 0.5000 and 0.0078 µg/mL (1.50×10−3 to 2.35×10−5 µM), and NOR between 2.0000 and 0.0312 µg/mL (6.27×10−3 to 9.78×10−5 µM), against S. aureus. In the assays with E. coli, the concentration of the solutions ranged from 0.0156 to 0.0002 µg/mL (4.70×10−5 to 6.02×10−7 µM) for CIP, and 1.250 to 0.019 µg/mL (3.92×10−3 to 5.96×10−5 µM) for NOR. The suspension of microorganisms was prepared in the same way as described for MIC determination. After 24 h of incubation at 35°C, the MIC was determined to be the minimal concentration of FQ at which there was no visible growth. To evaluate the effect of the combinations, the Fractional Inhibitory Concentration (FIC) was calculated for each antibiotic in each combination.36) The FIC index was calculated as:
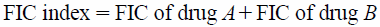 |
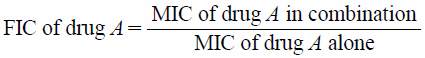 |
 |
The minimum FIC of the calculated FICs was defined to be the FIC index. The obtained values of the FIC index were assigned as follows: less than 0.5, synergism; between 0.5 and 1, partial synergism and between 1 and 4, indifference. Antagonism was defined as an FIC index greater than 4.33,34)
The time–kill method of synergy testing was performed by the broth macrodilution technique and following the guidelines of standardized methods.31) Each organism was tested against CIP and NOR alone and in various combinations. The bactericidal activity of the FQs studied, alone and in combination, was measured by determining viable counts. Time–kill studies were carried out in 100 mL flasks containing 50 mL of culture with the concentration of antibiotics described in Table 1: MIC CIP, MIC NOR and solutions A and B against S. aureus and MIC CIP, MIC NOR and solutions J to N against E. coli. These concentrations were selected on the basis of the results obtained previously using the checkerboard test. In this sense, the MIC of each FQ alone against each strain, and sub-inhibitory concentration of each compound in the combinations were assayed. Cells were grown to logarithmic phase, with 4 h of pre-incubation in fresh broth prior to the addition of drug. The starting bacterial density contained approximately 5.0×105 to 1.0×106 CFU/mL. Aliquots for the determination of viable counts were taken from test cultures and growth control flasks after 0, 2, 4, 6, 8, 11 and 24 h of drug additions. All assays were performed in duplicate. At different sample times, an aliquot of 0.1 mL was withdrawn from each tube and 10-fold dilutions were prepared when necessary. Viable counts were determined by plating on Mueller–Hinton agar by the method of Miles and Misra (20 µL delivered on each of five spots) or spread plate (100 µL on each of three plates). Cell count plates were incubated for up to 48 h at 35°C before any were considered as having no growth. Colonies were counted and averaged. The rate and extent of killing were determined by plotting viable colony counts (log10 CFU/mL) against time. The lower limit of detection for time–kill assays was 1.3 log10 CFU/mL.
| Organism | Combination | MIC relationship | Concentration combination | FIC index | Time–kill result | |
|---|---|---|---|---|---|---|
| µg/mL | µM | Value/interpretation | ||||
| S. aureus ATCC 29213 | MIC CIP | 0.2500 | 7.53×10−4 | — | — | |
| MIC NOR | 1.0000 | 3.13×10−3 | — | — | ||
| A | 1/4 MIC CIP+1/4 MIC NOR | 0.0625+0.2500 | 1.88×10−4+7.84×10−4 | 0.50 S | I/A | |
| B | 1/4 MIC CIP+1/16 MIC NOR | 0.0625+0.0625 | 1.88×10−4+1.99×10−4 | — | I/A | |
| C | 1/4 MIC NOR | 0.2500 | 7.84×10−4 | — | — | |
| D | MIC CIP+1/4 MIC NOR | 0.2500+0.2500 | 7.53×10−4+7.84×10−4 | — | — | |
| E | 2×MIC CIP | 0.5000 | 1.51×10−3 | — | — | |
| F | 1/2 MIC NOR | 0.5000 | 1.56×10−3 | — | — | |
| G | 2×MIC CIP+1/2 MIC NOR | 0.5000+0.5000 | 1.51×10−3+1.56×10−3 | — | — | |
| H | 1/4 MIC CIP | 0.0625 | 1.88×10−4 | — | — | |
| I | 1/16 MIC NOR | 0.0625 | 1.99×10−4 | — | — | |
| E. coli ATCC 25922 | MIC CIP | 0.0078 | 2.26×10−5 | — | — | |
| MIC NOR | 0.0625 | 1.96×10−4 | — | — | ||
| J | 1/2 MIC CIP+1/2 MIC NOR | 0.0037+0.0312 | 1.11×10−5+9.78×10−4 | — | S | |
| K | 1/4 MIC CIP+1/2 MIC NOR | 0.0018+0.0312 | 5.42×10−6+9.78×10−5 | — | I/A | |
| L | 1/8 MIC CIP+1/2 MIC NOR | 0.0009+0.0312 | 2.71×10−6+9.78×10−5 | 0.62 PS | I/A | |
| M | 1/2 MIC CIP+1/4 MIC NOR | 0.0037+0.0156 | 1.11×10−5+4.89×10−5 | — | I/A | |
| N | 1/2 MIC CIP+1/8 MIC NOR | 0.0037+0.0075 | 1.11×10−5+2.45×10−5 | 0.62 PS | I/A | |
S: synergism, PS: partial synergism, I/A: indifference/additivity.
The rate of bacterial killing from exposure to antimicrobial agents is interpreted as the time needed to achieve a 3-log10 reduction compared with the growth control. This parameter, determined from the time–kill curves, was assumed as an acceptable index of bactericidal activity.32) With this definition, the fact that an antimicrobial agent is considered as bactericide does not necessarily imply that there is no re-growth of colony count at longer times, but only that CFU is decreased by a factor of three orders of magnitude.
Synergy was defined as a ≥100-fold or 2−log10 decrease in colony count at 24 h by the combination compared with that by the most active single drug. Antagonism was defined as a ≥100-fold increase and additivity or indifference when any other values in-between the above mentioned were obtained.37)
To evaluate the generation of ROS in S. aureus ATCC 29213, involving an alteration of superoxide anion (O2−) production, the nitroblue tetrazolium (NBT) reduction test was performed.22) In this way, 0.1 mL of bacterial suspension (OD600 1.0) in Hanks’ balanced salt solution (HBSS) were incubated with 0.1 mL of drug solution and 0.5 mL of 1 mg/mL NBT for 30 min at 37°C. The concentrations of antibiotic employed were: MIC CIP, MIC NOR and solutions A to I (coming from combinations of FIC, MIC or MIC fractions) described in Table 1. Then, 0.1 mL of 0.1 M HCl was added to stop the reaction and the tubes were centrifuged at 1500×g for 10 min. The separated pellets were treated with 0.6 mL dimethylsulfoxide to extract the reduced NBT. Finally, 0.8 mL HBSS was added and optical density was determined at 575 nm.
As we already pointed out above, the structure of COP, along with the model proposed by Shen et al., prompted us to explore synergistic antibacterial activity between NOR and CIP. These profiles, expressed as MICs for S. aureus ATCC 29213 (Gram-positive) and E. coli ATCC 25922 (Gram-negative), are shown in Table 1. CIP showed better activity than NOR, and Gram-negative (E. coli) were more susceptible than Gram-positive bacteria (S. aureus) in accordance with previous reports.6,32–35) The impact of different mixtures of CIP and NOR on in vitro antibacterial activity of S. aureus ATCC 29213 or E. coli ATCC 25922 were evaluated, focusing on synergy, partial synergy and indifference. Checkerboard microdilution assays were performed and the FIC index was then calculated. All the combinations of CIP and NOR were more active against the last strain. Against S. aureus, one out of 49 assayed combinations resulted in a synergistic effect, with a FIC index=0.5, corresponding to 1/4 MIC of CIP and 1/4 MIC of NOR (combination A, Table 1). On the other hand, the effect against E. coli showed partial synergism, FIC index=0.620, at combinations of 1/8 MIC of CIP and 1/2 MIC of NOR, and 1/2 MIC of CIP and 1/8 MIC of NOR (combinations L and N, respectively, Table 1). Among all the assayed FQ combinations, no addition or antagonism was observed.
To confirm the synergistic activity between CIP and NOR, time–kill experiments were performed. Figure 2 shows that NOR at its MIC against S. aureus, demonstrated bactericidal activity at 8 h, with a viability of 5×105 CFU/mL (control=1×109 CFU/mL). CIP showed bactericidal activity at 24 h. Indeed, neither the combinations A and B (see Table 1) showed bactericidal activity at any time. When evaluating the time–kill curve at 24 h, it is possible to observe a 1−log10-fold change in colony count by the combinations compared to NOR, the most active single compound. Thus, against S. aureus, the effect of the assayed combinations is described as additivity or indifference (Fig. 2).

Growth control (◆); MIC CIP (■); MIC NOR (▲); combinations A (○) and B (□).
By a different way, during kinetics studies against E. coli, more than a 3−log10 reduction in CFU/mL at MIC concentration of CIP and NOR and at all the assayed combinations, was observed in less than 4 h (Fig. 3). Results obtained at 24 h demonstrated synergistic activity by the mixture 1/2 MIC CIP+1/2 MIC NOR (combination J, Table 1), with a 3−log10 decrease in colony count compared to CIP or NOR alone (Fig. 3). Furthermore, indifference or additivity of the mixtures K–N (see combinations in Table 1) was observed.

Growth control (◆); MIC CIP (■); MIC NOR (▲); combinations J (×), K (*), L (□), M (+) and N (−).
The activity of antimicrobial combinations could be assessed in vitro using checkerboard and time–kill methods, opinion with which most microbiologists would agree.31) Nevertheless, neither of these two methods is without limitations. Furthermore, comparison of results between them is not possible due to variations in techniques and the criteria to classify the interactions.6,37,38) It should be noted that the checkerboard method assesses bacteriostatic activity only, while time–kill studies test both bacteriostatic and bactericidal activities. This discrepancy has been documented by different investigators.38,39) The lack of correlation between the checkerboard and killing-curve methods is also observed in this study. Nevertheless, the checkerboard method was used with the aim of selecting adequate concentrations to be assayed by time–kill studies.
In relation to ROS generation in presence of antibacterial substances, and the impact over the strains, Dwyer et al. have provided significant evidences that different classes of bactericidal antibiotics, regardless of their drug-target interactions, induce complex redox alterations and generate varying levels of deleterious ROS that contribute to cellular damage and death.40) In accordance with this, Belenky et al.41) described that NOR affect the central carbon metabolism, oxidative phosphorylation and nucleotide metabolism. They observed a striking decrease in nucleoside, nucleotide, and purine/pyrimidine base levels in response to antibiotic treatment, suggestive of a diminishing pool of nucleotide building blocks. This decrease, coupled with increased xanthine levels, supports the notion that NOR may accelerate nucleotide turnover, which may be indicative of higher levels of DNA damage.41)
Kottur and Nair observed that the reduction in cell survival upon NOR treatment therefore occurs in part due to an increase in the oxidized nucleotide pool within the cell, and this can only occur due to an increase in ROS level. So, diverse activities of NOR contribute to cell death, and the ability to induce ROS represents one of the major mechanisms to generate cell lethality.42) In regard to CIP, Becerra et al. communicated that this bactericidal compound has the capacity of generates high oxidative stress in S. aureus and E. coli, with increased production of O2− which could affect lipid, DNA, and others components of bacterial cell, triggering subsequent cellular injury.22,23) Masadeh et al. published that the ROS generated by CIP are singlet oxygen (1O2) and superoxide anion (O2−) and reported that this effect is inhibited by the pretreatment of bacteria with antioxidants agents such as tempol, melatonin, pentoxifylline or vitamins E and C.43)
Moreover, Saveta Popa et al. evaluated the ability of NOR and CIP to induce lipid peroxidation and hydroxyl radical formation in rat, following acute administration, and concluded that minor structural differences can lead to different metabolic pathways and differences of toxicity between members of the same therapeutic class. NOR can form a metabolite capable of redox cycling and generating highly toxic hydroxyl radicals, while CIP can induce lipid peroxidation.44)
Some studies were conducted to evaluate the impact of synergistic mixtures of antibiotics over the generation of ROS. In this sense, Barnes et al. described that the combination of ampicillin and gentamicin generated an important synergistic increase in ROS in gentamicin-resistant strains of E. faecalis.45) By another way, Molina-Quiroz et al. demonstrated that synergistic combination of tellurite and cefotaxime caused increased levels of intracellular superoxide and OH·, generating direct damage to DNA (and probably RNA) and to the protein pool in E. coli.46)
In this work, the effect of equimolecular and not equimolecular combinations of NOR and CIP on the S. aureus ATCC 29213 ROS generation, was evaluated by measuring superoxide anion (O2−) production. In this sense, we observed that the combination D of CIP and NOR (Table 1) induced more ROS than the drugs alone (MIC CIP and C in Fig. 4) in the same concentrations. By another way, the combination B (Table 1) showed higher effect than NOR alone (solution F, Table 1) or than CIP alone (solution H, Table 1). These results indicate that the equimolar combinations of CIP and NOR assayed (combinations D and B) generated higher levels of ROS than the respective fractions of MICs employed. By another way, combinations not equimolecular of CIP and NOR assayed, generated less levels of ROS than the components alone and, particularly, the combination A (FIC index=0.50) generated a level of ROS (0.16 D.O.) a little minor of the 1/4 MIC CIP (0.20 D.O.) and 1/4 MIC NOR (0.22 D.O.). This is a very significant result, since just those combinations coincident with the COP stoichiometry were found to be more favourable for inducing radical species than CIP or NOR alone. This could be explained by the different capacity of NOR and CIP to generate ROS species.22,23,41,42) To date, no direct relationships between antibacterial agent concentration and ROS, MIC and ROS or combinations of antibacterial agents and ROS have been published for this strain. Furthermore, we should take into account that the ROS generation is one of the causes, among others, of the complex and multifactorial antibacterial effect.47)

Statistical significance was determined using t-test, ** p<0.01.
This is the first time that results of the effects of combinations of CIP and NOR against ROS are published. A more detailed understanding of them implies penetrating in deeper investigations at the molecular level, which is a future project.
Moreover, it is reassuring to note that the findings described herein are in accordance with results obtained by using checkerboard and time–kill methodologies to assay synergism.
The in vitro antibacterial activity against S. aureus ATCC 29213 and E. coli 25922 of all tested combinations of CIP and NOR showed synergism, partial synergism or indifference, when accepted methods of this type of research were performed. Neither of them showed antagonism.
By another way, against S. aureus, equimolecular combinations of CIP and NOR generated upper levels of ROS than the drugs alone in the same concentration and combinations not equimolecular of CIP and NOR assayed, generated less levels of ROS than the components alone. Until this moment, it is not known a direct relationship between antibacterial agent concentration and ROS, MIC and ROS or combinations of antibacterial and ROS in this strain. Deeper investigations at the molecular level are needed, which is the reason of future projects.
Although not necessarily associated a priori, when microbiological findings are analyzed along with those previously reported for a solid multi-component molecular complex, the tendency of CIP and NOR for a cooperative behavior was evident, this behavior being similar to that proposed by Shen et al. for DNA-gyrase inhibition.
The synergistic effect demonstrated by a combination of CIP and NOR against E. coli is promising from a biopharmaceutical point of view. To translate the results described herein to a possible clinical use of combinations of these antibiotics, the only requirement to be overcome should be bioequivalence and bioavailability studies, owing to the fact that CIP and NOR are approved drugs. Dissolution test assays are in progress.
This work was supported by Grant BID 802/OC-AR PICT 0361 from ANPCyT and SECyT of the Universidad Nacional de Córdoba. MRC is grateful to the University of Cape Town and the NRF (Pretoria) for Research support. MCB is a career member of CONICET. We thank Roux Ocefa S.A. (Buenos Aires, Argentina) and Finadiet (Buenos Aires, Argentina) for providing ciprofloxacin and norfloxacin, respectively. Special thanks to Prof. Inés Albesa, Ph.D. for her contribution to the discussion of part of this work.
The authors declare no conflict of interest.