2017 Volume 40 Issue 7 Pages 947-953
2017 Volume 40 Issue 7 Pages 947-953
Chemotherapy-induced peripheral neuropathy (CIPN), characterized by symptoms of paresthesia, dysesthesia, numbness, and pain, is a common adverse effect of several chemotherapeutic agents, including platinum-based agents, taxanes, and vinca alkaloids. However, no effective prevention or treatment strategies exist for CIPN because the mechanisms underpinning this neuropathy are poorly understood. Recent accumulating evidence suggests that some transient receptor potential (TRP) channels functioning as nociceptors in primary sensory neurons are responsible for CIPN. In this review, we focus on the specific roles of redox-sensitive TRP ankyrin 1 (TRPA1), which was first reported to be a cold nociceptor, in acute cold hypersensitivity induced by oxaliplatin, a platinum-based agent, because it induces a peculiar cold-triggered CIPN during or within hours after its infusion. Oxaliplatin-induced rapid-onset cold hypersensitivity is ameliorated by TRPA1 blockade or deficiency in mice. Consistent with this, oxaliplatin enhances the responsiveness of TRPA1 stimulation, but not of TRP melastatin 8 (TRPM8) and TRP vanilloid 1 (TRPV1), in mice and cultured mouse dorsal root ganglion neurons. These responses are mimicked by an oxaliplatin metabolite, oxalate. In human TRPA1 (hTRPA1)-expressing cells, oxaliplatin or oxalate causes TRPA1 sensitization to reactive oxygen species (ROS) by inhibiting prolyl hydroxylases (PHDs). Inhibition of PHD-mediated hydroxylation of a proline residue within the N-terminal ankyrin repeat of hTRPA1 endows TRPA1 with cold sensitivity by its sensing of cold-evoked ROS. This review discusses these findings and summarizes the evidence demonstrating that oxaliplatin-induced acute cold hypersensitivity is caused by TRPA1 sensitization to ROS via PHD inhibition, which enables TRPA1 to convert ROS signaling into cold sensitivity.
Chemotherapy-induced peripheral neuropathy (CIPN) is a frequent and disabling adverse effect of several chemotherapeutic agents, such as platinum-based agents (cisplatin, oxaliplatin, and carboplatin), taxanes (paclitaxel and docetaxel), vinca alkaloids (vincristine and vinblastine), and a proteasome inhibitor (bortezomib). Sensory symptoms include paresthesia, dysesthesia, numbness, and/or pain, which usually start in the hands, feet, or both; chronic CIPN is sometimes associated with motor and autonomic dysfunction.1–4) The incidence, as well as the severity, of CIPN depends on several factors, including the drug administered, patient age, cumulative dose, treatment duration, concomitant drugs, and medical conditions, such as diabetes or alcohol abuse, but is estimated to be from 30 to 40% of patients receiving these chemotherapeutics.1–4) Thus, CIPN impacts the quality of life of patients with cancer and cancer survivors and often results in chemotherapeutic dose delay or reduction or in treatment discontinuation.5,6)
Many clinical trials have been conducted to investigate potential prevention or treatment of CIPN.7) One phase III randomized, double-blind, placebo-controlled crossover clinical trial showed a significant reduction in painful CIPN following administration of the serotonin–noradrenaline reuptake inhibitor duloxetine,8) which is recommended as first-line treatment for neuropathic pain.9) The American Society of Clinical Oncology Clinical Practice guidelines recommend duloxetine as the best available treatment option for painful CIPN, although it has limited efficacy for painful CIPN and is largely ineffective in treating sensory loss or functional disability.10) To date, there is no effective strategy for preventing the occurrence of CIPN, which can become chronic and persist from months to years after chemotherapy termination.10)
A better understanding of the molecular and cellular mechanisms is required to develop novel therapeutic strategies for preventing or treating CIPN. In addition to chemotherapeutic agent-induced DNA damage and microtubule assembly inhibition, a large body of experimental evidence suggests that mitochondrial dysfunction, excess reactive oxygen species (ROS) production, ion channel alteration, and intracellular Ca2+ dysregulation trigger neurotoxicity in peripheral neurons.11,12) Furthermore, neuroinflammation mediated through accumulated inflammatory cells in the dorsal root ganglion (DRG) and spinal glial cells causes peripheral and central sensitization of nociceptive neurons, which may underlie painful CIPN.12,13) In this review, we focus on the roles of transient receptor potential (TRP) channels in CIPN, and in particular on oxaliplatin-induced acute CIPN.
TRP family proteins form tetrameric non-selective cation channels that detect a variety of extracellular and intracellular stimuli. Activation of TRP channels induces depolarization of the membrane potential and regulates Ca2+ signaling, which controls diverse cellular functions. Mammalian TRP channels are currently divided into six subfamilies, TRP ankyrin (TRPA), TRP canonical (TRPC), TRP melastatin (TRPM), TRP melastatin-like (TRPML), TRP polycystic (TRPP), and TRP vanilloid (TRPV), and 27 members have been characterized.14,15) Among them, some TRP channels belonging to the TRPV, TRPM, and TRPA subfamilies have been well documented as being thermosensitive in the range of physiological temperatures.14–16) Several types of thermosensitive TRP channels are expressed mainly in primary sensory neurons and act as polymodal nociceptors in response to a variety of thermal, chemical, and mechanical stimuli, leading to pain generation.17,18) For example, TRPV1 is activated by noxious heat (>42°C), acidity (pH <5.9), and noxious chemical stimuli, such as capsaicin.19,20) TRPV4 is activated by a wide variety of physical and chemical stimuli, including hypotonicity, heat (>25°C), and endocannabinoids.21) TRPM8 is activated by innocuous cold (<27°C) and by menthol, the ingredient in peppermint that produces its cooling sensation.22,23) TRPA1 is activated by noxious cold (<17°C) in rodents (but see the discussion below) and by a large number of irritant chemicals, including allyl isothiocyanate (AITC), cinnamaldehyde, allicin, and aldehydes, as well as by oxidative stimuli, such as ROS and hyperoxia, and by hypoxia.24–28) TRPA1 is focused as a novel target for treating pathological pain and respiratory disorders, because it is expressed abundantly on the nociceptive C fibers that innervate skin and respiratory tract from oral cavity and oropharynx.29)
Recent evidence suggests that these TRP channels play a crucial role in CIPN. In rodents, repeated administration of paclitaxel, an inhibitor of microtubule depolymerization, induces mechanical allodynia to application of von Frey filaments and thermal hyperalgesia to heat stimulation. The paclitaxel-induced painful symptoms are inhibited by TRPV1, TRPA1, or TRPV4 antagonists or gene deficiencies.30–32) Paclitaxel upregulates TRPV1 expression in small and medium DRG neurons, that is, nociceptive sensory neurons,33) and leads to sensitization of these TRP channels through proteinase-activated receptor 2 or Toll-like receptor 4 signaling.31,34) Similarly, repeated administration of cisplatin, a classic platinum anticancer agent, increases the expression levels of TRPV1 and TRPA1 in the mouse trigeminal ganglia, and cisplatin-induced thermal hyperalgesia is inhibited in TRPV1-knockout (KO) mice.35) Interestingly, TRPV1 is essential for hearing loss, a peculiar adverse effect induced by cisplatin.36) Mechanical and cold allodynia induced by bortezomib, a proteasome inhibitor, is attenuated by a TRPA1 antagonist.37) The involvement of TRP channels has been particularly well investigated in painful CIPN induced by oxaliplatin, a third-generation platinum anticancer agent, as described below.
Oxaliplatin is frequently used for locally advanced and metastatic cancer of the colon or rectum. It has a better safety profile, characterized by lower hematotoxicity and manageable gastrointestinal toxicity, than the classic platinum agent cisplatin. Unlike other chemotherapeutic agents, oxaliplatin induces a peculiar acute CIPN in approximately 90% of patients during or within hours after its infusion. Acute CIPN is specific to oxaliplatin and is often triggered or exacerbated by exposure to cold, inducing typical symptoms, including cold hypersensitivity, throat discomfort, paresthesia and dysesthesia of the hands, feet, and perioral region, which limit activities of daily living in patients. It usually improves after a few days, while it does not completely resolve between oxaliplatin doses. After multiple chemotherapy cycles, cumulative and chronic CIPN, such as sensory loss and motor dysfunction, occur in 10–15% of treated patients.1–4,38) Patients with the most severe acute symptoms are at higher risk to develop more severe chronic neuropathy.39)
Most behavioral studies using rodent models of oxaliplatin-induced painful CIPN have focused on the subacute and/or chronic phase that appears several days to several weeks after single or repeated administration of oxaliplatin.35,40–43) By contrast, oxaliplatin-induced acute CIPN is poorly characterized.44) We previously showed that a single intraperitoneal administration of oxaliplatin in mice produces rapid-onset cold hypersensitivity as assessed using a cold-plate test (at 5°C) even 2 h after administration, which is consistent with the clinical observation that characteristic acute CIPN is triggered by cold during or within hours of oxaliplatin infusion. However, administration of a single dose of oxaliplatin induces mechanical hypersensitivity 1 d, but not as early as 2 h, after drug administration and this hypersensitivity persists for at least 7 d. In contrast to oxaliplatin, neither cisplatin nor paclitaxel produces rapid-onset cold hypersensitivity. Thus, it is possible that rapid-onset cold hypersensitivity is representative of an acute CIPN characteristic to oxaliplatin administration in mice.45) The effects of standard analgesics and analgesic adjuvants on oxaliplatin-induced acute cold hypersensitivity were assessed in mice.46) The calcium channel α2-δ subunit ligand gabapentin and the voltage-gated Na+ channel blocker mexiletine strongly inhibit acute cold hypersensitivity, while the inhibitory effects of the opioid morphine and the serotonin–noradrenaline reuptake inhibitor milnacipran are weak. The non-steroidal anti-inflammatory agent diclofenac and the tricyclic antidepressant amitriptyline have no effect on acute cold hypersensitivity. Although it is difficult to clearly interpret these results, analgesics with an ability to directly suppress the hyperexcitability of sensory neurons may have higher efficacy than centrally acting analgesics. Nevertheless, such unusual drug sensitivity supports the possibility that the oxaliplatin-induced cold hypersensitivity observed in mice may represent cold-triggered dysesthesia as a clinical symptom of CIPN, rather than pain.
The mechanisms underlying oxaliplatin-induced CIPN have been extensively investigated in animal models.11,12,47,48) The chronic phase of oxaliplatin-induced CIPN is explained, at least in part, by primary sensory neuron neurotoxicity induced via mitochondrial dysfunction and ROS production,41) following accumulation of platinum in the DRG.46) Oxaliplatin is physiochemically and nonenzymatically metabolized to the platinum-containing metabolite dichloro(1,2-diaminocyclohexane)platinum(II) (Pt(DACH)Cl2) and the leaving group oxalate. In contrast to oxaliplatin-induced chronic CIPN, it has been proposed that oxaliplatin-specific acute CIPN is caused by oxalate, which chelates Ca2+ ions. Sakurai et al. demonstrated that oxalate contributes to the early-phase cold hyperalgesia, but not the late-phase mechanical allodynia, induced by oxaliplatin in rats.44) Oxaliplatin-specific acute CIPN is recognized as a channelopathy. A body of evidence suggests that oxaliplatin transiently alters the kinetics of axonal voltage-gated Na+ channels49–52) and blocks voltage-gated K+ channels.53) Oxalate can alter the voltage-gated Na+ channels by chelating Ca2+ ions.54)
Because oxaliplatin-induced acute CIPN is triggered or enhanced by cold, studies have focused on the involvement of cold-sensitive TRP channels, such as TRPA1 and TRPM8. Despite some controversy, many studies report that the mechanical and cold hypersensitivity induced by single or repeated administration of oxaliplatin is abolished by pharmacological inhibition or a gene deficiency of TRPA1 or TRPM8.35,40–42) Furthermore, oxaliplatin increases the mRNA expression levels of TRPA1 and TRPM8,35,40–42) but not of TRPV1,35) in the DRG. Goshajinkigan, which is a traditional Japanese medicine expected to have clinical efficacy against oxaliplatin-induced CIPN,55) reduces oxaliplatin-enhanced nociceptive behaviors evoked by TRPA1 or TRPM8 stimulation in rodents56) and decreases the upregulation of TRPA1 or TRPM8 mRNA expression in the rat DRG.57,58) These findings suggest the involvement of TRPA1 and TRPM8 in oxaliplatin-induced subacute and/or chronic CIPN.
Oxaliplatin-induced rapid-onset cold hypersensitivity is abolished by a TRPA1 antagonist and in TRPA1-KO mice.45) Furthermore, pretreating mice with oxaliplatin or its metabolite oxalate for 2 h enhances the nociceptive behaviors evoked by intraplantar injections of the TRPA1 agonist AITC, whereas neither cisplatin nor paclitaxel affects AITC-evoked nocifensive behaviors. These results suggest that TRPA1 contributes to oxaliplatin-induced rapid-onset cold hypersensitivity.
To further confirm the involvement of thermosensitive TRP channels, Ca2+ imaging experiments were performed to assess the effects of oxaliplatin pretreatment on the responses to TRPA1, TRPM8, and TRPV1 agonists (AITC, menthol, and capsaicin, respectively) in cultured mouse DRG neurons.45) Pretreatment of cultured mouse DRG neurons with oxaliplatin for 1–4 h increases the number of AITC-sensitive neurons, whereas there is no change in the number of menthol- or capsaicin-sensitive neurons. As described above, it has been reported that subacute (several days) and chronic (several weeks) administration of oxaliplatin increases TRPA1 mRNA levels in the DRG,35,40) but it is unlikely that oxaliplatin increases the expression of functional TRPA1 protein within only several hours of administration. Consequently, it is suggested that a brief treatment with oxaliplatin preferentially produces an enhanced responsiveness of TRPA1, that is, TRPA1 sensitization, but not of TRPM8 and TRPV1.45) TRPA1 sensitization on sensory neurons may contribute to the typical symptoms of oxaliplatin-induced acute CIPN, such as hypersensitivity to touching cold items and discomfort swallowing cold items.
The TRPA1 channel can be opened by a variety of irritants26,59,60) and oxidative stimuli, such as ROS27) and hyperoxia.28) Activation of TRPA1 is caused by reversible covalent or oxidative modifications of the cysteine residues at the N-terminus of TRPA1.59–62) However, another mechanism for TRPA1 activation has been reported in which a decrease in oxygen concentration inhibits the activity of prolyl hydroxylases (PHDs).28) This inhibition relieves TRPA1 from the PHD-dependent hydroxylation of a proline residue located within the N-terminal ankyrin repeat domain (Pro394 in human TRPA1 [hTRPA1]), leading to hypoxia-induced opening of TRPA1. The 1-carboxylate and 2-oxo functional groups of oxalate are also common to a PHD cosubstrate, α-ketoglutarate, and the artificial PHD inhibitor dimethyloxalylglycine (DMOG).63) Thus, it is hypothesized that PHD inhibition-mediated TRPA1 activation may be responsible for oxaliplatin-induced rapid-onset cold hypersensitivity.
To determine whether oxaliplatin and oxalate inhibit PHD enzymatic activity, the amount of hypoxia-inducible factor 1α (HIF-1α), a molecule whose degradation is principally regulated by PHDs,64) were examined. Treatment with oxaliplatin or the membrane-permeable oxalate analog dimethyl oxalate (DMO) increases the amount of HIF-1α, suggesting that oxaliplatin or oxalate can suppress PHD activity.65) Ca2+ imaging experiments using hTRPA1-expressing HEK293 cells revealed that pretreatment with oxaliplatin or DMO for 2 h enhances sensitivity of TRPA1 to a relatively low concentration of hydrogen peroxide (H2O2), whereas pretreatment with another oxaliplatin metabolite, Pt(DACH)Cl2, has no effect. Similarly, pretreatment with the PHD inhibitor DMOG for 2 h also induces TRPA1 sensitization to ROS. Furthermore, overexpression of recombinant human PHD2 or a catalytically inactive dominant negative mutant of human PHD2 with hTRPA1 abolishes oxaliplatin-induced TRPA1 sensitization. In cells expressing mutant hTRPA1 lacking a hydroxylation-susceptible Pro394 residue (hTRPA1-P394A), oxaliplatin, DMO, and DMOG fail to further enhance the sensitivity of TRPA1 to H2O2. Taken together, these results suggest that oxaliplatin and DMO can induce TRPA1 sensitization to ROS by inhibiting the PHD-mediated hydroxylation of the Pro394 residue on hTRPA1.65)
Similar results were obtained in cultured mouse DRG neurons prepared from wild-type mice. By contrast, no DRG neurons prepared from TRPA1-KO mice respond to H2O2 even following pretreatment with oxaliplatin or DMO, suggesting that these agents selectively enhance the sensitivity of TRPA1 to ROS, but not to other ROS-sensitive channels, in DRG neurons.65)
Furthermore, it is confirmed that oxaliplatin can induce TRPA1 sensitization to ROS in vivo through PHD inhibition. Pre-administration of oxaliplatin, DMO, or DMOG facilitates the nocifensive behaviors evoked by an intraplantar injection of H2O2, and the enhanced response is suppressed by a TRPA1 antagonist. However, pre-administration of Pt(DACH)Cl2 has no effect, suggesting that the enhanced TRPA1-mediated nocifensive behavior induced by oxaliplatin is mediated through oxalate-induced PHD inhibition, but not through its platinum metabolite.65)
TRPA1 was first reported to be sensitive to noxious cold (<17°C),26) and some studies show that TRPA1 functions as a cold nociceptor both in vitro and in vivo.66–68) However, results from some early studies disagree with those from subsequent studies examining the cold sensitivity of TRPA1, and it is still a matter of debate whether TRPA1 is sensitive26,66–68) or insensitive59,60,69,70) to cold. Recent studies clearly demonstrate a species-specific difference in temperature sensitivity of TRPA1: TRPA1 in mouse and rat is activated by cold, but TRPA1 in human and rhesus monkey is cold-insensitive.71) Nevertheless, a recent study indicates that a gain-of-function mutation in the hTRPA1 gene identified it as responsible for the autosomal-dominant familial episodic pain syndrome triggered by fasting and physical stress, including cold.72) Furthermore, hTRPA1 purified and reconstituted into lipid bilayers is intrinsically cold-sensitive.73)
Based on the clinical feature that oxaliplatin-induced acute CIPN is triggered by cold, it is hypothesized that inhibiting PHD confers cold sensitivity on cold-insensitive hTRPA1 by enhancing sensitivity to ROS. Consistent with a previous report,71) whole-cell patch-clamp recordings and Ca2+ imaging experiments reveal that hTRPA1 is insensitive to cold stimulation (from 26 to 16°C), although mouse TRPA1 is intrinsically cold-sensitive. However, we found that mutant hTRPA1 lacking a hydroxylation-susceptible Pro394 residue (hTRPA1-P394A) shows cold sensitivity. Similar results were obtained in wild-type hTRPA1-expressing cells pretreated with the PHD inhibitor DMOG for 2 h. Because cold stimulation evokes mitochondria-derived ROS generation,74) the involvement of cold-induced ROS production in the cold sensitivity of hTRPA1 was explored. The experiments using PG-1, a fluorescent probe with high selectivity for H2O2 revealed that cold stimulation induces H2O2 production, which is suppressed by pretreatment with a mitochondria-targeted antioxidant mitoTEMPO. Furthermore, pretreatment with mitoTEMPO suppresses the cold-evoked activation both in DMOG-pretreated hTRPA1 and in hTRPA1-P394A.65) These results demonstrate that relieving hTRPA1 from prolyl hydroxylation enables the channel to sense cold by detecting cold-evoked ROS production. Similarly, the cold sensitivity of mouse TRPA1 is also enhanced by pretreatment with DMOG; this enhancement is suppressed by a ROS scavenger.65)
Cold-plate assays using mice were also performed to determine whether the in vitro findings were consistent with this in vivo mechanism of TRPA1 cold sensitivity. Pre-administration of DMOG or oxaliplatin increases cold-escape behavior, which is attenuated by a TRPA1 antagonist or a ROS scavenger. These results suggest that PHD inhibition, as well as oxaliplatin, is sufficient to evoke cold hypersensitivity, which is dependent on TRPA1 and ROS signaling.65)
It is speculated (although it remains a matter for debate) that the mechanisms underlying CIPN include the neurotoxicity of peripheral neurons caused by ROS-mediated mitochondrial dysfunction, axonal degeneration, demyelination, and subsequent peripheral and spinal neuroinflammatory responses.11–13) In this review, we summarize the evidence for a potential molecular mechanism underlying oxaliplatin-induced acute CIPN. Oxaliplatin or one of its metabolites, oxalate, can inhibit the PHD-mediated hydroxylation of an N-terminal proline residue of TRPA1, which induces TRPA1 sensitization to ROS and endows TRPA1 with cold sensitivity via transduction of ROS signaling (Fig. 1). Thus, chemical modification allows hTRPA1 to be activated by cold via its sensing of cold-induced ROS production. Recently, it is reported that spontaneous painful dysesthesia-like behaviors evoked by transient hindlimb ischemia and reperfusion are also caused by ROS-evoked activation of TRPA1 sensitized by hypoxia-induced PHD inhibition.75) Considering these results together, we conclude here that PHD inhibition-induced TRPA1 sensitization is commonly responsible for the dysesthesia (that is, an unpleasant abnormal sensation) induced by oxaliplatin, as well as transient hindlimb ischemia/reperfusion. Thus, the experimental evidence summarized in this review proposes that prophylactic TRPA1 blockade may provide clinical benefits for preventing oxaliplatin-induced acute CIPN, which may improve activities of daily living of the patient treated with oxaliplatin and also may prevent the progression to chronic CIPN.
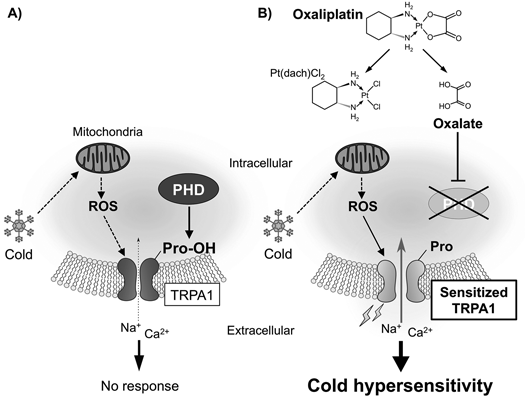
(A) Cold stimulation induces ROS production in mitochondria, but it is insufficient to activate TRPA1 with conserved PHD-mediated hydroxylation of a proline residue. (B) Oxaliplatin or one of its metabolites, oxalate, inhibits PHD activity and thus the PHD-mediated hydroxylation of an N-terminal proline residue on TRPA1, which induces TRPA1 sensitization to ROS. This enhanced sensitivity enables TRPA1 to sense cold-evoked ROS, which causes oxaliplatin-induced cold hypersensitivity.
This work was supported in part by Grants-in-Aid for Scientific Research (KAKENHI) from the Japanese Society for the Promotion of Science (Grants-in-Aid for Scientific Research (B) to T.N. [26293019], Challenging Exploratory Research to T.N. [15K14961], and Scientific Research on Innovative Area ‘Thermal Biology’ to T.N. [16H01386]), and by Grants from the Salt Science Research Foundation (No. 14C4) and the Nakatomi Foundation.
The authors declare no conflict of interest.