2017 Volume 40 Issue 9 Pages 1544-1550
2017 Volume 40 Issue 9 Pages 1544-1550
Alnus japonica (Betulaceae) is a broad-leaved tree easily found in damp regions within the mountains of Korea and Japan. Four triterpenoids (1–4) from the fruits of A. japonica, including the newly isolated 3β-hydroxy-lanost-9(11),23(24)-dien-25,26-diol (3), inhibited the lipopolysaccharide (LPS)-induced expression of the chemotactic cytokine interleukin (IL)-8 and nitric oxide (NO) production in HT-29 colon epithelial cells and RAW264.7 macrophages, respectively. Among these triterpenoids, compound 4, which showed the most potent inhibitory activity, effectively down-regulated LPS-induced protein expression of inducible nitric oxide synthase (iNOS) in RAW264.7 cells and in HT-29 cells. Also, compound 4 concentration-dependently inhibited the levels of LPS-induced pro-inflammatory cytokines, IL-1β and IL-6 in macrophage cells. These triterpenoids isolated from A. japonica fruits are thought to contribute to the anti-inflammatory activities of macrophage and colon epithelial cells, which are important for regulating the colon immune system. They are expected to be potential candidates for therapeutic agents against inflammatory bowel disease.
Inflammation is an important defense response against body tissue injuries and invading pathogens. Acute inflammation is a necessary reaction of the innate immune system to protect the host from a variety of etiologic agents such as chemical injuries, thermal and mechanical stress, pathogens, antigens, and infections.1,2) In contradistinction to acute inflammation, continuous stimulation by etiologic agents extending up to months or years cause excess levels of inflammatory mediators in macrophages.3) This is called the chronic inflammatory response, which may induce a variety of diseases including inflammatory bowel disease (IBD).4) Inflammatory bowel diseases including ulcerative colitis (UC) and Crohn’s disease (CD) are chronic relapsing disorders of the gastrointestinal tract characterized pathologically by epithelial injury and intestinal inflammation.5) The definite etiology of IBD remains unclear, but recent reports indicate that the innate immune system is crucial in the pathology of intestinal inflammation.4,6)
Cytokines play a pivotal role in the regulation of the intestinal immune system. The levels of various pro-inflammatory cytokines are elevated in patients with IBD.7) These cytokines are quickly stimulated and secreted by inflammatory cells, and have an effect on the induction, amplification, prolongation, and termination of inflammation.7) Nitric oxide (NO) and inducible nitric oxide synthase (iNOS) are main factors in the inflammatory process. NO helps macrophages with immunological activity rebuff etiologic agents.4) iNOS is an enzyme that produces NO when induced by lipopolysaccharide (LPS) or various pro-inflammatory cytokines and a high level of human iNOS is measured in macrophages from patients who have infections or inflammatory diseases.8) Therefore, down-regulation of the production of NO and iNOS may thus suppress or prevent a variety of inflammatory diseases. The production of pro-inflammatory cytokines such as interleukin (IL)-1β and IL-6 leads to acute and chronic inflammatory diseases and is stimulated by the activation of nuclear factor κB (NF-κB), which is involved in the regulation of many inflammation-associated genes. IL-8 is secreted from the intestinal epithelium against pathogenic infections and various stimuli in the early stages of inflammation, and an increase in IL-8 has been observed in the intestinal tissue of IBD patients.9) As the intestinal inflammatory mediator in IBD, macrophages as well as intestinal epithelial cells play a significant role in the intestinal immune response. Among the primary mediators of chronic inflammation such as reactive oxygen species, hydrolytic enzymes, cytokines and other growth factors, we focused on evaluating the expression of inflammatory cytokines such as IL-1β, IL-6, IL-8, and iNOS related to NO production.10)
The Alnus japonica STEUD. (Betulaceae) is a broad-leaved tree easily found in damp regions in the mountain valleys of Korea and Japan. A. japonica have been used as a traditional medicine to treat alcoholism, diarrhea, hemorrhage, antipyretic fever and burn injuries.11,12) There have been many studies on A. japonica demonstrating anti-tumor, anti-inflammatory, anti-oxidative, and anti-obesity effects.13) However, anti-inflammatory activity associated with IBD have not been investigated yet. As part of our ongoing screening program to evaluate the anti-inflammatory potential of natural compounds, we investigated the in vitro anti-inflammatory activities of A. japonica. This led to the isolation of four triterpenoids, which were evaluated for the down-regulation of LPS-stimulated inflammatory response in macrophages, RAW264.7 cells and colon epithelial HT-29 cells.
The A. japonica fruits were collected in the Nambu forest of Seoul, Beagwoon Mountain, Gwangyang city, Jeollanam-do, Korea. These Alnus species were identified by Dr. Jong Hee Park, a professor at Pusan National University. A voucher specimen (SCNUP003-F1) was deposited at the laboratory of Pharmacognosy, College of Pharmacy, Sunchon National University, Korea.
Extraction and IsolationThe fruits of A. japonica (1.5 kg) were dried, smashed and extracted three times with 80% methanol (4 L) for 3 h using ultrasonication. The residue was concentrated in vacuo to yield crude extract (419.1 g). The extract was suspended in H2O and fractioned in a regular sequence with n-hexane (4 L), CHCl3 (4 L), n-BuOH (4 L) and H2O to obtain residues of 29.5, 47.6, 251.6, and 62.7 g, respectively. Among these fractions, the CHCl3 fractions showed a significant inhibition effect on LPS-induced NO production in RAW264.7 cell. These fractions were used for further isolation work. The CHCl3 fraction was used for silica gel column chromatography using a gradient of CHCl3–MeOH–H2O to obtain fifteen fractions (C1–C15). C4 was subjected to medium pressure liquid chromatography (MPLC) (RediSep silica gel; CHCl3–MeOH, 15 : 1→MeOH, 30 mL/min) to obtain six fractions (C4-1–C4-6). Compound 1 (4.1 mg) was obtained from C4-3 by column chromatography on the Sephadex LH-20 (CHCl3–MeOH, 1 : 1). C4-5 was used for MPLC (RediSep silica gel; CHCl3→MeOH; 22 mL/min) to obtain seven fractions (C4-5-1–C4-5-7). C4-5-6 was used for preparative HPLC (polymeric gel filtration, 500×20 mm, n-hexane–EtOAc 3 : 1→1 : 1, 4 mL/min) to obtain thirty fractions (C4-5-6-1–C4-5-6-30). Compounds 2–4 were obtained by recrystallization from C4-5-6-7, C4-5-6-9, and C4-5-6-10, respectively.
3β-Hydroxy-lanost-9(11),23(24)-dien-25,26-diol (3), whitish amorphous powder; mp (°C): 203.5–205.5; [α]D25 +40.3 (c=1.0 CHCl3–MeOH=1 : 1); IR (KBr) νmax (cm−1): 3402, 2938, 1714, 1456, 1375, 1045; 1H-NMR (500 MHz, C5D6N) and 13C-NMR (125 MHz, C5D6N): Table 1; High resolution (HR)-FAB-MS (negative mode): m/z 457.0828 [M−H]− (Calcd for C30H49O3, 457.0826).
| Position | δH (J in Hz) | δC |
|---|---|---|
| 1 | 1.81, 1.52, m | 36.8, t |
| 2 | 1.94, m | 28.7, t |
| 3 | 3.43, m | 78.0, d |
| 4 | 39.7, s | |
| 5 | 0.99, m | 53.0, d |
| 6 | 1.72, 1.54, m | 21.8, t |
| 7 | 1.63, 1.21, m | 28.5, t |
| 8 | 2.20, m | 42.1, d |
| 9 | 149.2, s | |
| 10 | 39.8, s | |
| 11 | 5.27, d (5.5) | 115.0, d |
| 12 | 2.05, 1.89 m | 37.4, t |
| 13 | 44.6, s | |
| 14 | 47.3, s | |
| 15 | 1.40, m | 34.1, t |
| 16 | 1.27, m | 28.3, t |
| 17 | 1.65, m | 51.5, d |
| 18 | 0.69, s | 14.7, q |
| 19 | 1.12, s | 22.6, q |
| 20 | 1.54, m | 36.8, d |
| 21 | 0.96, d (6.3) | 18.7, q |
| 22 | 2.30, 1.89, m | 39.7, t |
| 23 | 6.12, td (15.5, 8.1) | 127.0, d |
| 24 | 6.00, d (15.5) | 138.2, d |
| 25 | 73.2, s | |
| 26 | 3.95, d (10.5) | 71.2, t |
| 3.90, d (10.5) | ||
| 27 | 1.65, s | 25.5, s |
| 28 | 1.29, s | 28.9, q |
| 29 | 1.07, s | 16.5, q |
| 30 | 0.77, s | 18.6, q |
1H- and 13C-NMR data were measured at 500 and 125 MHz in C5D6N, respectively. Multiplicity was determined by DEPT experiments.
Human colonic epithelial cells (HT-29) and mouse macrophage cells (RAW264.7) were acquired from the Korean Cell lines bank (Seoul, Korea). These cells were cultured as monolayers in a 37°C humidified atmosphere with 5% CO2. The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Hyclone, U.S.A.) with 10% heat-inactivated fetal bovine serum (FBS) (Hyclone, U.S.A.) containing 100 µg/mL streptomycin and 100 IU/mL penicillin. In the present study, four triterpenoids at final concentrations of 5–25 µM were added to HT-29 cells and RAW264.7 cells for 1 h and the cells were treated with LPS (1 µg/mL) for 24 h. Cell viability and NO production were determined with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Dojindo, Japan) and Griess reagent, respectively.14)
Expression of Pro-inflammatory Cytokines14)The level of IL-8 was determined using enzyme-linked immunosorbent assay (ELISA) kits (BD OptEIA™, CA, U.S.A.) in HT-29 human colonic epithelial cells. The levels of IL-1β and IL-6 were detected by mouse ELISA kits (Cusabio, Wuhan, China) in RAW264.7 mouse macrophage cells.
Western BlottingCells were lysed with lysis buffer (50 mM Tris, pH 7.4, 1 mM ethylenediaminetetraacetic acid (EDTA), 0.1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride (PMSF), 25 µg/mL leupeptin, and 20 µg/mL pepstatin) on ice for 20 min. After centrifugation at 12000×g for 20 min, the protein concentration in the supernatant was determined. Protein samples were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA, U.S.A.). The membranes were blocked with TBST [10 mM Tris (pH 7.4), 100 mM NaCl, and 0.5% Tween 20] containing 5% bovine serum albumin (BSA) for 30 min at RT and incubated with anti-iNOS antibody (1 : 1000, Cell Signaling, MA, U.S.A.) in TBST containing 1% milk powder overnight at 4°C. Then, the membranes were incubated with horseradish peroxidase (HRP) with conjugated anti-mouse-immunoglobulin G (IgG) (1 : 1000, Santa Cruz, CA, U.S.A.). The membranes were visualized by enhanced chemiluminescence (ECL-kit, Thermo, U.S.A.) and detected using a Bio imaging-system (MicroChemi 4.2 Chemilumineszenz-System, Israel).
Statistical AnalysisThe evaluation of statistical significance was determined by a “one-way ANOVA” test using a computerized statistical package. All data were expressed as means±standard deviation (S.D.) of at least three independent experiments, and statistical significance was shown at p<0.05.
The increase of inflammatory mediators in the inflamed mucosa indicates that the activation of the intestinal immune system plays a central role in the pathophysiology of chronic mucosal inflammation and the associated clinical symptoms.5) Mucosal and systemic concentrations of pro-inflammatory cytokines are elevated in IBD.7) To discover more anti-inflammatory candidates, we investigated natural products, which are used as traditional medicines and have attracted attention, due to the growing interest in their health benefits. In the course of searching for anti-inflammatory natural product, we further investigated the A. japonica fruit, which was previously reported to have the anti-adipogenic activities based on studying the diarylheptanoids isolated from the extract fractions.15) In the present study, we attempted to isolate other phytochemicals, with the goal of preventing IBD, and screened the anti-inflammatory effects of the constituents isolated from A. japonica employing RAW264.7 and HT-29 cells as an in vitro assay system. The four triterpenoids (1–4) were obtained from the CHCl3 fraction of 80% methanolic extract of A. japonica fruits were on successive normal phase column chromatography using a multi-gradient as the mobile phase (Fig. 1).

Compound 3 was isolated as a whitish amorphous powder, [α]D25 +40.3 (c=1.0 CHCl3–MeOH=1 : 1). The molecular formula was determined to be C30H50O3 from the negative HR-FAB-MS at m/z 457.0828 [M−H]− (Calcd for C30H49O3, 457.0826). The 1H-NMR spectrum of compound 3 showed evidence of the presence of seven tert-CH3 groups [δH 1.65 (3H, s, H-27), 1.29 (3H, s, H-28), 1.12 (3H, s, H-19), 1.07 (3H, s, H-29), 0.77 (3H, s, H-30), 0.69 (3H, s, H-18)], two olefinic double bonds [δH 5.27 (1H, br d, J=5.5 Hz, H-11), 6.12 (1H, td, J=15.5, 8.1 Hz, H-23), 6.00 (1H, d, J=15.5 Hz, H-24)], geminal protons [δH 3.95 (1H, d, J=10.5 Hz, H-26) and 3.90 (1H, d, J=10.5 Hz, H-26)]. The J-value (15.5 Hz) between H-23 and H-24 indicated that the olefinic bond was trans-type. The 13C-NMR spectrum exhibited 30 carbon signals sorted by distortionless enhancement by polarization transfer (DEPT) experiment as seven tert-CH3 groups [δC 28.9 (C-28), 22.6 (C-19), 18.7 (C-21), 18.6 (C-30), 16.5 (C-29), 14.7 (C-18)], eight methylenes including one oxygenated carbon [δC 71.2 (C-26), 39.7 (C-22), 37.4 (C-12), 36.8 (C-1), 34.1 (C-15), 28.7 (C-2), 28.5 (C-7), 28.3 (C-16), 21.8 (C-6)], eight hybridized methines including one oxygenated carbon and three olefinic carbons [δC 138.2 (C-24), 127.0 (C-23), 115.0 (C-11), 78.0 (C-3), 53.0 (C-5), 51.5 (C-17), 42.1 (C-8), 36.8 (C-20)], five quaternary sp3-carbons [δC 73.2 (C-25), 47.3 (C-14), 44.6 (C-13), 39.7 (C-4), 25.5 (C-27)], and one quaternary sp2-carbon [δC 149.2 (C-9)], suggesting a tetracyclic triterpenoid. The location of hydroxyl groups was evidenced by the relative downfield signals of H-3, H-26 and heteronuclear multiple bond correlation (HMBC) correlations: H-3 to C-28/C-29; H-26 to C-25; H-23/H-24 to C-25 (Fig. 2). The 1H–1H correlation spectroscopy (COSY) and heteronuclear single quantum correlation (HSQC) experiments revealed the following key fragments: CH2–CH(OH)– (A); C(C)=CH–CH2– (B); CH(CH3)–CH–CH2–CH=CH– (C) (Fig. 2). Taking into account all of the above described spectroscopic data, the compound was considered a lanostane type triterpene with hydroxyl and olefinic moieties. In the nuclear Overhauser effect spectroscopy (NOESY) spectrum, the cross peaks observed between CH3-18 and H-8, and between CH3-19 and H-8 as well as the lack of a cross peak between H-5 and H-8/CH3-19 further supported the lanostane-type skeleton (Fig. 2). On the basis of these data, compound 3 was determined to be 3β-hydroxy-lanost-9(11),23(24)-dien-25,26-diol, isolated for the first time from nature.
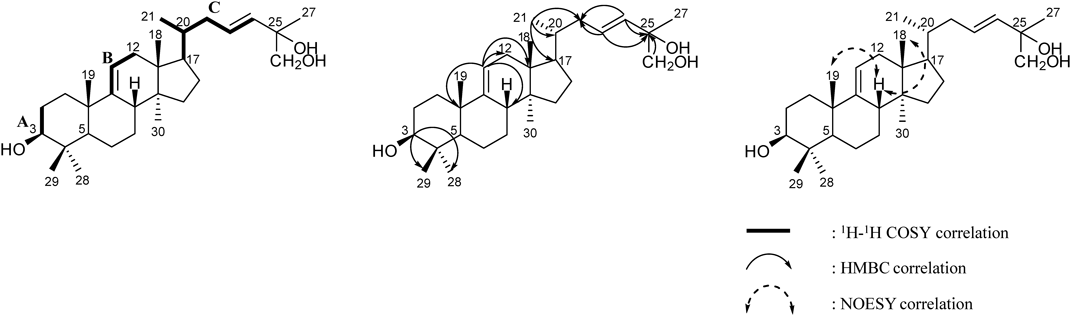
The three known compounds were identified as 3β-hydroxy-lanost-9(11),24(25)-dien-26-oic acid (1), (24S)-lanost-9(11)-ene-3,24,25-triol (2), and leucastrin B (4) by comparing the measured spectroscopic data with published values.16–18)
We evaluated the effects of the triterpenoids isolated from A. japonica fruits on inflammatory responses using the cell line models of macrophage and colon epithelial cells that can mimic the intestinal epithelial cell condition during IBD.19)
Using HT-29 cells, we measured the effects of the four triterpenoids isolated from A. japonica by applying various concentrations (5–25 µM) to the cells for 1 h, incubating with 1 µg/mL LPS for 24 h, followed by MTT assay. The LPS-treated groups and sample-treated groups showed 92.4% and over 85% cell viability compared to the non-treated control group, respectively (Fig. 3A). HT-29 cells secrete substantial amounts of IL-8 when exposed to LPS.20) Normal intestinal epithelial cells have the potential to secret the potent chemoattractant IL-8 and might contribute to inflammation as opposed to normal mucosa.20) The inhibitors of the pro-inflammatory chemokine, IL-8, may be used for the treatment of immune-associated diseases, IBD and thus, we assessed the LPS-stimulated IL-8 expression of triterpenoids in HT-29 cells. Compounds 1, 2, and 4 effectively decreased the expression of IL-8, compared to curcumin, while compound 3 had no effect (Fig. 3B). Especially, compound 4 considerably attenuated IL-8 expression below the control level at a concentration of 25 µM.
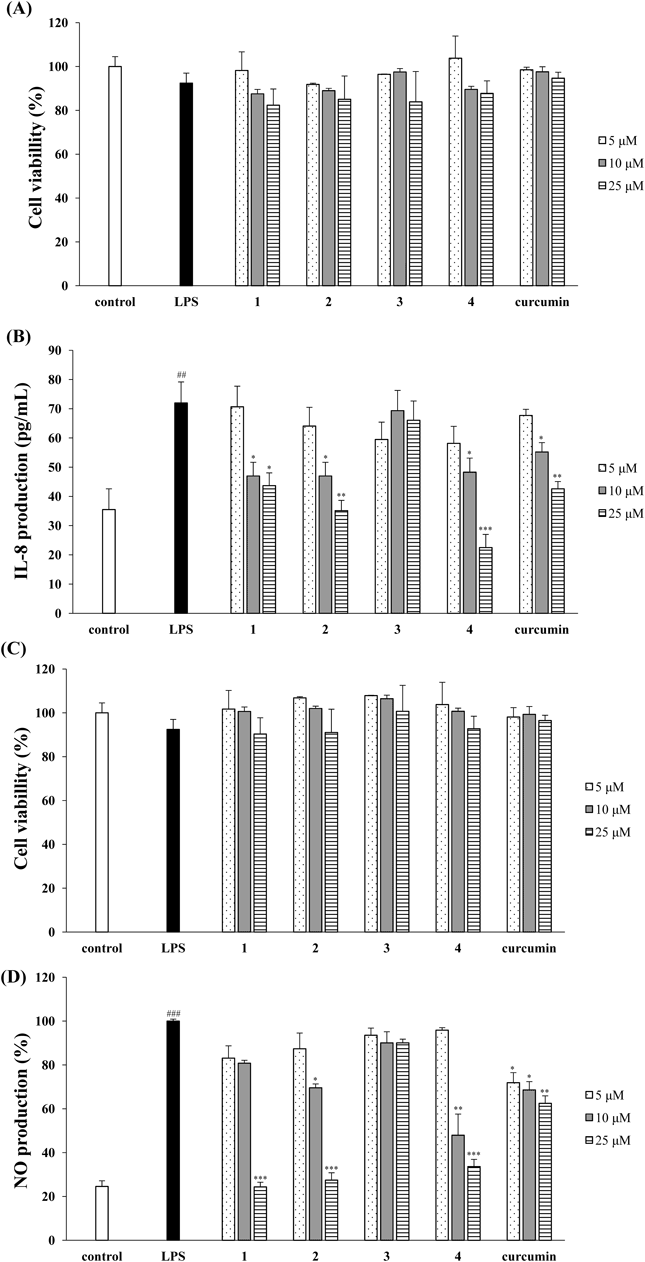
HT 29 cells and RAW264.7 cells were pretreated in the presence of different concentrations of compound 4, then stimulated with 1 µg/mL LPS for 24 h. (A, C) The cells viability was determinded with the MTT assay. (B) IL-8 level in culture media was measured with an ELISA kit. (D) The NO production in culture medium was measured using Griess reagents. Curcumin was used as the positive control. The values are expressed as the mean±S.D. (n=3) of three individual experiments. ## p<0.01 and ### p<0.001, compared with untreated control; * p<0.05, ** p<0.01 and *** p<0.001, compared with LPS-treated control.
RAW264.7 cells were treated with four triterpenoids for 1 h, then cultured with 1 µg/mL LPS for 24 h followed by the MTT assay. The four triterpenoids exerted no significant cytotoxicity at concentrations up to 25 µM (Fig. 3C). The production of NO, a pleiotropic free radical messenger molecule, is promoted in human IBD tissues.21) NO is produced by iNOS, which is up-regulated in various forms of mucosal inflammation.21) The production of excess NO and inflammatory mediators is associated with inflammatory process. It has been reported that natural compounds have suppressive effects on NO and the secretion of inflammatory mediators for the development of therapeutic agents to treat inflammatory diseases.22,23) Compounds 1, 2, and 4 concentration-dependently decreased NO production in comparison to the LPS-treated group (Fig. 3D). Compound 3 exerted no effect on NO production and seemed to hardly have any anti-inflammatory activity. At a concentration of 10 µM, compound 4 considerably suppressed NO production up to 47.9%, whereas compounds 1, 2, and curcumin attenuated NO production up to 80.8, 69.6, and 68.6%, respectively.
From the results in Fig. 3, we suggest that compound 4 has the most potent anti-inflammatory effect in both LPS-stimulated RAW264.7 cells and HT-29 cells among the four triterpenoids isolated from A. japonica. It has been reported that iNOS is necessary for the production of NO. In order to investigate the effects of compound 4 on the expression of LPS-induced inflammatory proteins, iNOS were evaluated in RAW264.7 cells and HT-29 cells. As showed in Fig. 4, LPS treatment without compound 4 significantly increased the expression of iNOS protein, while pretreatment of compound 4 exhibited a concentration-dependent inhibition of the LPS-induced expression of this protein.
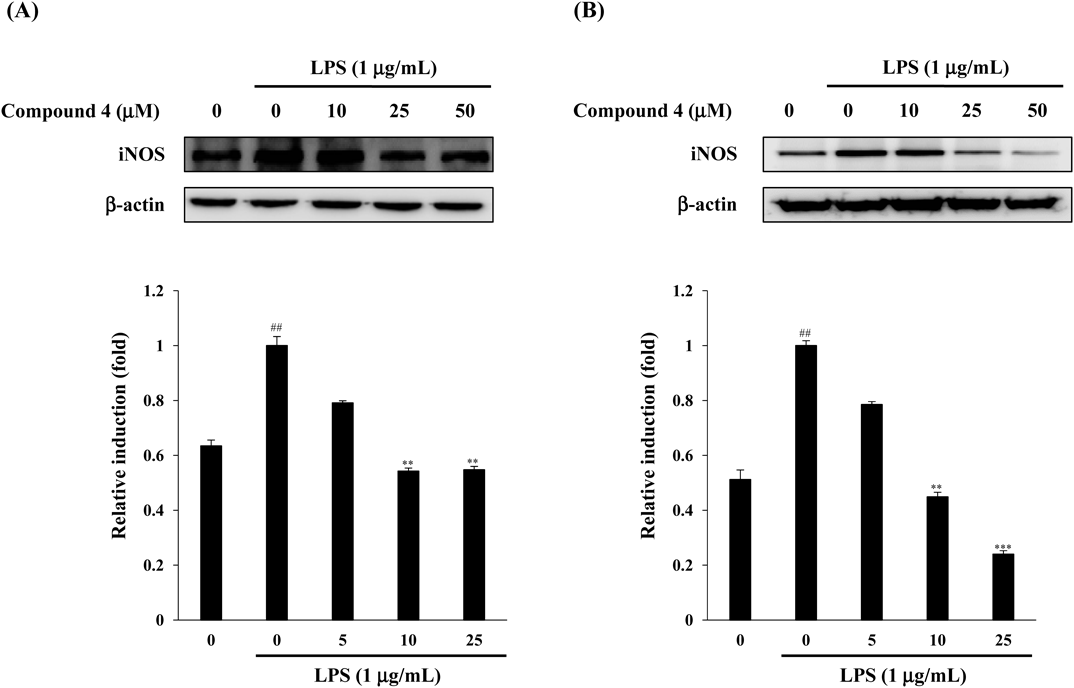
Cells were pretreated with the indicated concentration of compound 4 for 1 h and stimulated with LPS (1 µg/mL) for 24 h. The expression of iNOS and β-actin was determined by Western blot analysis using specific antibodies. The values are expressed as the mean±S.D. (n=3) of three individual experiments. ## p<0.01, compared with untreated control; ** p<0.01 and *** p<0.001, compared with untreated control.
Activated macrophages and colon epithelial cells produce pro-inflammatory cytokines such as IL-1β and IL-6. To confirm the anti-inflammatory activity of compound 4, we measured the LPS-induced production of IL-1β and IL-6 using an ELISA kit. IL-1β and IL-6 production was significantly inhibited by compound 4 at concentrations ranging from 5 µM to 25 µM (Fig. 5). Mahida et al. demonstrated that LPS elevates the production of IL-1β in mononuclear cells from active IBD mucosa but not those from normal mucosa.24) Increased IL-6 expression by lamina propria macrophages has been observed in experimental colitis and in IBD patients.5,25) The suppression of IL-6 signaling with monoclonal antibodies was effective in the inhibition of chronic intestinal inflammation in mouse models, which suggests IL-6 as a potential therapeutic target in IBD.5) Likewise, proinflammatory cytokines, IL-1β and IL-6, are amplified in the IBD-affected intestine.26) High secretion of mucosal IL-1β in IBD could be caused by infiltrating macrophages, which have been found to migrate in large numbers into the inflamed mucosa and the intestinal lumen during UC and CD.25) Furthermore, IL-1β can enhance the secretion of potent chemotactic cytokines, IL-8 by stimulating different cell types, and IL-8 elevates the migration of granulocytes into the intestinal mucosa.26)

Cells pretreated with various concentrations of compound 4 for 1 h were stimulated with LPS (1 µg/mL) for 24 h. The amount of cytokines IL-1β (A) and IL-6 (B) in the culture medium was measured using manual ELISA kits, as described in Materials and Methods. The values are expressed as the mean±S.D. (n=3) of three individual experiments. ### p<0.01, compared with untreated control; * p<0.05, ** p<0.01 and *** p<0.001, compared with LPS-treated control.
This work was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korea government (MSIP) (No. NRF-2017R1C1B2005934). This work was supported by Suncheon Research Center for Natural Medicines.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials. Refer to Web version on Pubmed Central for supplementary material.