2017 Volume 40 Issue 9 Pages 1561-1565
2017 Volume 40 Issue 9 Pages 1561-1565
Human intestinal absorption and drug metabolism vary to a large extent among individuals. For example, CYP3A4 activity has large individual variation that cannot be attributed to only genetic differences. Various flavonoids in vegetables, such as kaempferol and quercetin, possess inhibitory effects, and some vegetable and fruit juices have also been found to inhibit CYP3A4 activity. Therefore, differences in daily intake of flavonoid-containing vegetables may induce individual variation in intestinal bioavailability. To identify a vegetable that strongly inhibits CYP3A4, we investigated the effects of juices, prepared from individual vegetables, on CYP3A4 activity using recombinant CYP3A4 and LS180 cells in this study. Nine vegetable juices (cabbage, Japanese radish, onion, tomato, eggplant, carrot, Chinese cabbage, green pepper, and lettuce), were prepared and recombinant CYP3A4 and LS180 cells were used for evaluation of CYP3A4 activity. Metabolism to 6β-hydroxytestosterone by recombinant CYP3A4 was strongly inhibited by cabbage, onion, and green pepper juices, and cabbage and green pepper juices significantly inhibited CYP3A4 activity in a preincubation time-dependent manner. In addition, CYP3A4 activity in LS180 cells was significantly inhibited by cabbage and onion juices. In conclusion, this study showed that juices prepared from some individual vegetables could significantly inhibit CYP3A4 activity. Therefore, variation in the daily intake of vegetables such as cabbage and onion may be one of the factors responsible for individual differences in intestinal bioavailability.
Human intestinal absorption and drug metabolism are known to have a large degree of variation among individuals. For example, CYP3A4 activity has considerable individual variation that cannot be attributed to only genetic differences such as CYP2C19 polymorphisms.1) Therefore, acquired factors such as eating habits might be responsible for individual variation in intestinal bioavailability (absorption and/or metabolism).
Flavonoids, which are contained in various vegetables and fruits,2,3) have anti-oxidative properties,4,5) and many flavonoid-containing beverages such as lime and grapefruit juices have been observed to increase the intestinal absorption of CYP3A4 substrates such as felodipne and nisoljipine in clinical studies.6,7) In addition, we have reported that various flavonoids inhibit CYP3A4 activity in human liver microsomes.8) These reports indicate that differences in the daily intake of flavonoid-containing foods and beverages may be the cause of individual variation in intestinal drug absorption.
Additionally, we previously reported that some vegetables and fruit juices inhibit CYP3A4 activity using human recombinant CYP3A4 and the intestinal human colon adenocarcinoma cell line LS-180.9) Juices prepared using orange, green, and yellow vegetables, including bell pepper, cabbage, carrot, Chinese cabbage, eggplant, Japanese radish, and lettuce, strongly inhibited CYP3A4 activity. These vegetables contain various flavonoids such as kaempferol and quercetin that can inhibit CYP3A4 activity.8) These reports suggest that juices prepared using individual vegetables can inhibit CYP3A4 activity, and differences in the daily intake of flavonoid-containing vegetables may induce individual variation in intestinal bioavailability. Some researchers have reported that flavonoid-containing fruit juices,10) teas,11) and traditional medicine12) can inhibit CYP3A4 activity, but the effects of typical vegetables that most people consume almost every day remain unclear. To identify vegetable juices that strongly inhibit CYP3A4, we investigated the effects of the juices on CYP3A4 activity using recombinant CYP3A4 and LS180 cells in this study.
Nicotinamide adenine dinucleotide phosphate (NADP+), glucose-6-phosphate (G6P), glucose-6-phosphate dehydrogenase (G6PDH), testosterone, ketoconazole, and carbamazepine were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Human CYP3A4+P450 reductase+Cytochrome b5 Supersomes were purchased from BD Biosciences (Woburn, MA, U.S.A.). 6β-hydroxytestosterone was purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, U.S.A.). All other chemicals were reagent- or HPLC-grade commercial products.
Sample PreparationCabbage (Brassica oleracea var. capitata, Tokushima, Japan), Japanese radish (Raphanus sativus var. longipinnatus, Shiga, Japan), onion (Allium cepa, Hokkaido, Japan), tomato (Solanum lycopersicum, Mie, Japan), eggplant (Solanum melongena, Okayama, Japan), Chinese cabbage (Brassica rapa var. pekinensis, Nagasaki, Japan), green bell pepper (Capsicum annuum L. var. grossum, Kochi, Japan), and lettuce (Lactuca sativa, Hyogo, Japan) were selected as representative vegetables in this study. These vegetables are consumed by Japanese people frequently and often used for preparation of commercially available vegetable juices. All vegetables were juiced using a juice extractor (AMX-100, Alphax Koizumi Corp., Saitama, Japan). The soluble materials were almost perfectly contained in the juice and the insoluble residue was almost completely removed. The juices were centrifuged (1630×g, 10 min) and then filtered using filter papers and 0.45-µm membrane filters (Merck Millipore, Billerica, MA, U.S.A.) to remove the insoluble materials perfectly. Juice samples were described by weight per volume percent (w/v%), obtained by dividing the vegetable weight before compression by the volume of the experimental buffer. The reason why the volumes of the juice samples were described by vegetable weight before compression and not the solution volume of the vegetable juices is that the volume of the juice that can be obtained from the vegetables varies depending on the vegetable (cabbage, 0.37 mL/g; Japanese radish, 0.65 mL/g; onion, 0.62 mL/g; tomato, 0.28 mL/g; eggplant, 0.30 mL/g; carrot, 0.42 mL/g; Chinese cabbage, 0.79 mL/g; green bell pepper, 0.65 mL/g; lettuce, 0.69 mL/g). All vegetables juices were frozen at −30°C until the experiment.
In the ethyl acetate extraction experiment, ethyl acetate was added to vegetable juices at 4-fold their volume, and the mixture was vortexed at 300 strokes/min for 15 min. After centrifugation at 1630×g for 10 min, the upper layer was collected and evaporated to dryness at 40°C under a stream of nitrogen. The residue was then dissolved in dimethyl sulfoxide.
Detection of CYP3A4 Activity in Human Recombinant CYP3A4Inhibitory experiments in human recombinant CYP3A4 were performed following a method previously reported by us.9) In addition, the 6β-hydroxytestosterone assay was conducted using the HPLC-UV method, as previously reported by us.9)
Detection of CYP3A4 Activity in LS180 Cells Using the P450-Glo-Assay KitHuman colon adenocarcinoma LS180 cells (American Type Culture Collection, Manassas, VA, U.S.A.) were selected for in vitro evaluation of CYP3A4 activity. CYP3A4 activity was measured using the P450-Glo assay (Luciferin-IPA selectively reacts with CYP3A4 and hardly reacts with CYP3A5 and CYP3A7: Promega, Madison, WI, U.S.A.), as previously reported by us.9)
Estimation of Pharmacokinetic ParametersTo calculate the pharmacokinetic parameters, namely, the Michaelis constant (Km), maximum velocity (Vmax), and IC50, of testosterone metabolism by human recombinant CYP3A4, Eqs. 1 and 2 were fitted to the observed data by non-linear least-squares regression analysis with the MULTI program13):
 | (1) |
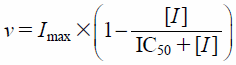 | (2) |
All data are presented as the mean±standard error. Significant differences were determined using the unpaired Student’s t-test or ANOVA followed by Dunnett’s test. p<0.05 was considered statistically significant.
The metabolism to 6β-hydroxytestosterone by human CYP3A4 was strongly inhibited by cabbage, onion, and green pepper juices (15 w/v%) and slightly inhibited by Japanese radish, tomato, eggplant, carrot, Chinese cabbage, and lettuce juices (15 w/v%) (Fig. 1). In addition, all vegetables inhibited CYP3A4 activity in a concentration-dependent manner, and the approximate IC50 values were 7.21±0.94 (cabbage), 53.7±13.5 (Japanese radish), 0.216±0.019 (onion), 105±29 (tomato), 25.0±7.6 (eggplant), 11.6±1.6 (carrot), 45.8±8.8 (Chinese cabbage), 5.48±0.54 (green pepper), and 17.2±3.7 (lettuce) w/v% (Fig. 2). Cabbage (7.21 w/v%), onion (0.22 w/v%), and green pepper juices (5.48 w/v%) increased the Km value by approximately two-folds at approximately the IC50 value, and Vmax value was unchanged (Table 1).

The reaction was performed in an NADPH-generating system containing recombinant CYP3A4 and testosterone (10 µM) at 37°C for 10 min in the absence (control) or presence of vegetable juices (15 w/v%) or 50 µM ketoconazole (KTZ, positive control). Each column represents the mean±S.E. (n=3–4). Significance of differences between mean values was determined by Dunnett’s test (** p<0.01 vs. control). N.D.: Not detected.

The reaction was performed in an NADPH-generating system containing recombinant CYP3A4 and testosterone (10 µM) at 37°C for 10 min in the presence of vegetable samples (0.1–15 w/v%). Each point represents the mean±S.E. (n=3–4).
| Vmax (pmol·min−1·(pmol P450)−1) | Km (mM) | |
|---|---|---|
| Control | 0.155 (0.150–0.160) | 23.8 (21.3–26.2) |
| Cabbage | 0.156 (0.149–0.163) | 51.8 (45.3–58.2) |
| Onion | 0.144 (0.130–0.158) | 61.0 (46.0–76.0) |
| Green pepper | 0.163 (0.155–0.172) | 50.7 (44.0–57.4) |
Vmax and Km values for the formation of 6β-hydroxytestosterone were determined using recombinant CYP3A4. Vmax and Km values were calculated from Michaelis–Menten kinetics. Each value represents the estimates (95% confidence interval) (n=3–4).
Pretreatment of cabbage (7.21 w/v%) and green pepper juices (5.48 w/v%) but not onion juice (0.22 w/v%) inhibited CYP3A4 activity (Fig. 3). The inhibition of CYP3A4 activity by the ethyl acetate extract of cabbage juice (7.21 w/v%) was similar to that by the non-extracted cabbage juice (7.21 w/v%). However, inhibition of CYP3A4 activity by the ethyl acetate extract of onion juice (0.22 w/v%) was weaker than that by non-extracted onion juice. The ethyl acetate extract of green pepper (5.48 w/v%) did not inhibit CYP3A4 activity (Fig. 4). Cabbage (15 w/v%) and onion juices (15 w/v%) significantly inhibited CYP3A4 activity in LS180 cells, in contrast to green pepper (15 w/v%) juice (Fig. 5).

The reaction was performed in an NADPH-generating system with testosterone (10 µM) at 37°C for 10 min after pretreatment with recombinant CYP3A4 for 0 min (white column), 10 min (gray column), or 20 min (black column) in the absence (control) or presence of vegetable juices (7.21 w/v% cabbage, 0.22 w/v% onion, and 5.48 w/v% green pepper). Each column represents the mean±S.E. (n=7–8). Significance of differences between mean values was determined by Dunnett’s test (** p<0.01 vs. 0 min).

The reaction was performed in an NADPH-generating system containing recombinant CYP3A4 and testosterone (10 µM) at 37°C for 10 min in the absence (control) or presence of vegetable juices (7.21 w/v% cabbage, 0.22 w/v% onion, and 5.48 w/v% green pepper) not extracted (A) or extracted (B) with ethyl acetate. Each column represents the mean±S.E. (n=3–4). Significance of differences between mean values was determined by Dunnett’s test (* p<0.05 and ** p<0.01 vs. control).

LS180 cells were incubated with luciferin-IPA (3 µM) for 4 h in the absence (control) or presence of vegetable samples (15 w/v%) or 50 µM ketoconazole (KTZ, positive control). Luciferin detection reagent was added, and the plate was incubated at room temperature for 20 min. Each column represents the mean±S.E. (n=3–4). Significance of differences between mean values was determined by Dunnett’s test (** p<0.01 vs. control).
Some vegetable juices (15 w/v%) significantly inhibited metabolism of testosterone to 6β-hydroxytestosterone by human CYP3A4 (Fig. 1), and their IC50 values varied widely between 0.216 and 105 w/v% (Fig. 2), indicating that juices prepared from individual vegetables inhibited CYP3A4 activity. Strong inhibition of CYP3A4 activity was exhibited by cabbage, onion, and green pepper juices.
If the volume of the human gastrointestinal tract is assumed to be 1 L, the intake equivalents for each IC50 value are 72 g (cabbage), 538 g (Japanese radish), 2.2 g (onion), 1054 g (tomato), 250 g (eggplant), 116 g (carrot), 458 g (Chinese cabbage), 54 g (green pepper), and 172 g (lettuce). These amounts of cabbage, onion, and green pepper were assumed to be consumed in one meal. In addition, the treatment of cabbage, onion, and green pepper juices resulted in an increase in the Km value for CYP3A4 metabolism, but did not change the Vmax value (Table 1), and the pretreatment of cabbage and green pepper juices significantly decreased CYP3A4 activity (Fig. 3). These results suggest that cabbage, onion, and green pepper juices may competitively inhibit CYP3A4 activity, and that the inhibition by cabbage and green pepper juices may be irreversible. Therefore, differences in the daily consumption levels of these vegetables might partly explain the individual differences in CYP3A4 activity.
In this study, we found that onion juice strongly inhibited CYP3A4 activity using recombinant CYP3A4 (Fig. 2). Although ethyl acetate extraction slightly weakened the inhibitory effects of onion juice, even 0.22 w/v% onion juice inhibited CYP3A4 activity significantly (Fig. 4). In addition, onion juice (15 w/v%) significantly inhibited CYP3A4 activity in LS180 cells (Fig. 5). These results indicate the possible influence of the intake of onion on intestinal CYP3A4 functioning. We have already reported the strong inhibitory effects of quercetin, a major flavonoid present in onion, on CYP3A4 activity in human liver microsomes.8) In addition, because quercetin can be extracted by ethyl acetate14) and consequently may be present in the ethyl acetate extract of onion juice, it could play an important role in the inhibitory effects exhibited by onions.
Furthermore, this study showed that onion juice strongly inhibited CYP3A4 activity (Fig. 1) in a reversible manner (Fig. 3). Orange vegetable juices strongly inhibited CYP3A4 activity compared to other vegetable juices, but the inhibition was slightly time-dependent during preincubation.9) In addition, quercetin is known to be a reversible inhibitor of CYP3A4 activity.15) Therefore, quercetin contained in onion may be considered to be a major inhibitory component in the mixture of orange vegetables juices.
Most of the inhibitory effects of green pepper juice disappeared after ethyl acetate extraction (Fig. 4), and the extract did not inhibit CYP3A4 activity in LS180 cells (Fig. 5). We obtained similar results for green vegetable juice in our previous study.8) These results show that a water-soluble substance that cannot permeate the cell wall is involved in the inhibitory effect of green pepper. Therefore, green pepper consumption does not influence individual differences in CYP3A4 activity.
Ethyl acetate extraction did not affect the inhibitory effects of cabbage juice, which significantly inhibited CYP3A4 activity in LS180 cells (Fig. 5). However, quercetin occurs in low amounts in cabbage,1) and therefore, other substances that can permeate the cell wall may be involved in the inhibitory effects of cabbage.
The present study has several limitations. First, human colon adenocarcinoma LS180 cells, not normal human intestinal cells, were used in present study. However, it is thought that LS180 cells are useful because they have high expression of CYP3A4. Second, we could not consider the osmolality of the incubation medium with vegetable juices. Therefore, we cannot deny that osmotic pressure has influenced intracellular uptake of Luciferin-IPA. Third, the inhibitory effects of vegetable juices against CYP3A4 activity have not been tested in vivo. However, pretreatment with quercetin reportedly increases the bioavailability of pioglitazone, which is a substrate of CYP3A4, in rats.16) Therefore, onion juice at least could possibly inhibit CYP3A4 activity in vivo. Fourth, because the amount of flavonoids in vegetables varies across cultivation condition, we cannot conclude that all cabbage and onion can inhibit CYP3A4 activity. Future studies such as clinical trials involving substrates of CYP3A4 with or without consumption of onion- or cabbage-rich meals could solve this problem.
In conclusion, this study showed that some juices, prepared from individual vegetables, significantly inhibit CYP3A4 activity. Therefore, variation in the daily intake of vegetables such as cabbages and onions might be one of the causes for individual differences in intestinal bioavailability.
This study was supported financially in part by a Grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT)-Supported Program for the Strategic Research Foundation at Private Universities, 2012–2016 (S1201008).
The authors declare no conflict of interest.