2017 年 40 巻 9 号 p. 1566-1571
2017 年 40 巻 9 号 p. 1566-1571
The objective of this study was to evaluate the interactions between various drugs and aojiru (green juice), a popular health food in Japan, using a simple centrifugation method. The mixture of drug solution and aojiru suspension was gently shaken and centrifuged. The drug concentration in the supernatant fluid was then determined by HPLC. The concentration of rhodamine 123 (Rho-123), a model compound, in the supernatant fluid significantly decreased after mixing with aojiru, indicating extensive binding of Rho-123 to the insoluble components of aojiru. When administered into rat small intestinal loops together with aojiru, the plasma Rho-123 concentrations became much smaller than those when administered alone. This result strongly suggested that a strong interaction observed in vitro was well reflected in modulated absorption. Among seven drugs tested, chlorpromazine and imipramine exerted binding properties to aojiru similar to or greater than Rho-123. As a small part of both Rho-123 and imipramine was released when the aojiru precipitate was resuspended, their binding to aojiru was considered to be tight. The binding of diltiazem, fexofenadine, glibenclamide, metformin, and norfloxacin to aojiru was much weaker or almost negligible compared with that of chlorpromazine and imipramine. The present results suggest that aojiru can decrease the intestinal absorption of some clinically relevant drugs through tight binding in the small intestine and that the present centrifugation method is useful for predicting in vivo interactions between drugs and aojiru.
In Japan, with the recent increase in interest regarding health control, the use of health foods and dietary supplements has grown in getting popularity with patients as well as among the healthy population. However, limited information about the efficacy and safety of health foods and dietary supplements has been provided to consumers. Under such circumstances, unfavorable effects from the consumption of health foods and dietary supplements have been noted.1,2) Moreover, undesirable pharmacokinetic or pharmacodynamic interactions have been reported with various drugs.3–7) We also demonstrated that kurosu (Japanese unpolished rice vinegar) is capable of modulating the intestinal absorption of P-glycoprotein (P-gp) substrates.8) Accordingly, further accumulation of information about clinically relevant interactions between drugs and various kinds of health foods is required.
We previously reported the results of a questionnaire survey about the use of health foods among diabetic outpatients at the Health Sciences University of Hokkaido Hospital.9) The most frequently consumed health food was aojiru (green juice). Aojiru, one of the most popular health foods in Japan, is made from green vegetables such as kale, ashitaba, mulberry leaf and barley grass and considered useful as a dietary fibers supplement.10,11) However, aojiru products contain large amounts of vitamin K,12) which competes with warfarin. Aojiru also contains metallic cations such as calcium and magnesium, which chelate with fluoroquinolones and tetracyclines, leading to their impaired absorption.13) Furthermore, a variety of drugs tightly adsorb to dietary fiber,14) it is likely that dietary fiber including aojiru, modulate the absorption of various clinically relevant drugs through this adsorption.
Our previous questionnaire survey also demonstrated that metformin hydrochloride (MET) and sulfonylureas such as glibenclamide (GB) were frequently administered to patients taking aojiru and that about 20% among patients, who had any experience in ingesting health foods, took prescribed drugs together with health foods.15) As little information is available about the interactions between these antidiabetic drugs and aojiru, we investigated the binding characteristics of GB and MET to aojiru using a dialysis method and found that GB weakly but significantly interacted with aojiru in vitro.16)
Evaluation by dialysis method is normally based on the diffusion of a drug through a dialysis membrane. However, as the diffusion of drugs depends on their molecular weights (MW) and molecular shapes, the dialysis method is sometimes very time-consuming. In fact, in our previous study the outer medium concentrations of GB (MW: 494.0) and MET (MW: 165.5) were approximately 22 and 70% of the equilibrium concentration, respectively, after 90 min.16) Accordingly, it appears that the dialysis method is not always applicable for the rapid evaluation of interactions between aojiru and drugs. In this study, we investigated the interaction between aojiru and several drugs, using a simple centrifugation method, with special interest in whether the centrifugation method could serve for dialysis method.
Rho-123 was obtained from Acros Organics (Morris Plains, NJ, U.S.A.). GB, chlorpromazine hydrochloride (CP), diltiazem hydrochloride (DT) and norfloxacin (NFLX) were obtained from Wako Pure Chem. Ind. (Osaka, Japan). MET was purchased from BP Biomedical (Salon, OH, U.S.A.). Imipramine hydrochloride (IP) was purchased from Sigma Chem. Co. (St. Louis, MO, U.S.A.). Fexofenadine hydrochloride (FEX) was obtained from Toronto Research Chemicals (North York, Ontario, Canada). The structural formula of seven drugs and Rho-123 are presented in Fig. 1. A freeze-dried aojiru product made from kale was purchased from Fancl Co. (Yokohama, Japan). All other chemicals and reagents were of the highest grade available.

One pack (3.5 g) of aojiru powder was suspended in 100 mL of purified water. CP, DT, IP and MET were dissolved in purified water. The poorly water-soluble FEX, NFLX and Rho-123 were first dissolved in methanol or ethanol and then diluted to the experimental concentrations with purified water. In the case of GB, the dilution was done using a 2% solution of β-cyclodextrin, which is known to be a suitable solubilizing agent.17)
A solution of each drug or Rho-123 was mixed with an equal volume of aojiru suspension. The mixture (4 mL) was shaken in a stoppered test tube at 100 rpm for 30 min at room temperature and then centrifuged at 25900×g for 30 min at 25°C. The supernatant fluid obtained was filtered through a membrane filter (Millex-HV, pore size 0.45 µm, Millipore Co., Billerica, MA, U.S.A.) and the concentration in the filtrate was assayed. As control, a solution of each drug or Rho-123 was mixed with an equal volume of aojiru-free purified water and treated as described above. The final concentration of each drug or Rho-123 was 12.5, 25 or 50 µM, except NFLX, which did not dissolve at 50 µM in purified water, methanol or ethanol.
Further, the supernatant fluid was completely taken up in a syringe and the precipitate was again suspended with 4 mL of purified water and shaken at 100 rpm for 30 min at room temperature. The suspension was then centrifuged at 25900×g for 30 min at 25°C and the drug concentration in the supernatant fluid was determined after filtration.
Absorption ExperimentsIn the present study, principles of good laboratory animal care were followed and animal experimentation was performed in compliance with Guidelines for the Care and Use of Laboratory Animals in Health Sciences University of Hokkaido.
An in situ intestinal loop technique was used for absorption experiment as described previously18) with some modifications. Male Wistar rats (Hokudo, Sapporo, Japan) weighing ca. 350 g were anesthetized by sodium pentobarbital (intraperitoneally (i.p.), 40 mg/kg) after overnight fasting. An entire small intestinal loop (approximately 60 cm in length) was prepared by setting the upper ligature at 10 cm from the pylorus and the lower ligature at 10 cm above the ileocecal junction. Loop contents were washed out with 50 mL of saline, and 6 mL of Rho-123 solution, or its suspension with aojiru, was introduced into the loop from the upper side with a syringe. Just before administration and at 10, 30, 60, 90 and 120 min after administration, 0.6 mL of blood was collected from the jugular vein under anesthesia, transferred into test tubes containing a small volume of heparin sodium injection (Shimizu Pharmaceutical Co., Shizuoka, Japan), and immediately centrifuged at 6080×g for 15 min at 5°C. The plasma obtained was stored at −30°C until assay.
For assay of Rho-123, 0.1 mL of thawed plasma sample was mixed with 0.1 mL of purified water and 0.2 mL of methanol. After letting it stand for 10 min in iced water, the mixture was then centrifuged at 6080×g for 15 min at 5°C and the supernatant was used to determine Rho-123 concentration.
HPLC AnalysisRho-123 and all drugs except FEX were assayed using a Shimadzu LC-10A HPLC system (Shimadzu, Kyoto, Japan) equipped with a Shimadzu SPD-10A detector. FEX was assayed using a Shimadzu LC-10A HPLC system equipped with a Shimadzu RF-10AXL fluorescence detector. Chromatographic conditions were as follows: a Cosmosil 5C18-AR column (4.6×150 mm, Nacalai Tesque Inc., Kyoto, Japan) was used for CP, DT, GB, IP, MET and Rho-123, and an Inertsil ODS-3 column (4.6×250 mm) was used for FEX and NFLX; mobile phases were 0.05 M KH2PO4–CH3CN (1 : 1) for GB, 0.04 M NaH2PO4 containing 5 mM 1-octanesulfonic acid sodium salt (pH 6.0)–CH3CN (75 : 25) for MET, 0.05 M KH2PO4 containing 2% acetic acid–CH3CN (7 : 3, pH 5.0) for NFLX, 0.05 M KH2PO4 containing 0.2% triethylamine–CH3CN (6 : 4) for DT, and 0.05 M KH2PO4–CH3CN (6 : 4) for FEX, IP, CP and Rho-123; the wave length was 230 nm for GB, 234 nm for MET, 254 nm for IP and CP, 240 nm for DT, 273 nm for NFLX, 230 nm (excitation) and 280 nm (emission) for FEX, and 500 nm for Rho-123; the column temperature was 50°C for GB, MET, FEX, CP, NFLX and Rho-123, and 40°C for IP and DT, the flow rate was 1.0 mL/min for GB, MET, IP, CP and DT, 0.8 mL/min for NFLX and FEX, and 0.6 mL/min for Rho-123; and the injection volume was 20 µL.
Pharmacokinetic and Statistical AnalysisPharmacokinetic parameters of Rho-123 were calculated by MULTI, using a one-compartmental model.19) Area under the plasma concentration–time curves for 0 to 120 min (AUC0–120 min) was estimated by computer, using the linear trapezoidal rule. Data are expressed as the mean with standard error (S.E.) obtained from three to five experiments. Statistical comparisons were performed by Student’s t-test. The level of significance was set at p<0.05.
In our previous study investigating the effect of kurosu on drug absorption, Rho-123 was used as a model compound.8) So, we first evaluated whether Rho-123 was also useful for the present study. Figure 2 shows the interaction of Rho-123 with aojiru as evaluated using the centrifugation method. In the presence of aojiru, the Rho-123 concentration in the supernatant fluid obtained by centrifugation was approximately 55% lower than that for Rho-123 alone, indicating that Rho-123 extensively bound (or was adsorbed) to the insoluble components of the aojiru product used here.

Rho-123 solution was mixed with an aojiru suspension at concentrations of 12.5, 25, and 50 µM. Each column represents the mean with S.E. of 4 experiments. ** p<0.01, *** p<0.001, significantly different from Rho-123 alone.
An important focus in this study was to assess how in vitro interaction with aojiru observed using the centrifugation method was reflected in the intestinal absorption of Rho-123. To know it, Rho-123 was administered into the rat small intestinal loops with or without aojiru and its plasma concentrations were followed. As shown in Fig. 3, the plasma concentrations of Rho-123 administered together with aojiru were significantly lower than those without aojiru at all sampling time points. Several pharmacokinetic parameters are presented in Table 1. When Rho-123 was administered with aojiru, the Cmax value decreased significantly and the AUC0–120 min value fell markedly to just 37% of that for Rho-123 alone. Interestingly, the t1/2 value was significantly extended when Rho-123 was administered with aojiru (Table 1). From these results it was considered that the absorption of Rho-123 was markedly impaired due to its extensive binding to aojiru in the intestinal lumen and that the in vitro results on the potent interaction between Rho-123 and aojiru was clearly reflected in the absorption of Rho-123 in the rat small intestine. This implied that the present centrifugation method is useful for predicting in vivo interaction between various drugs and aojiru conveniently.
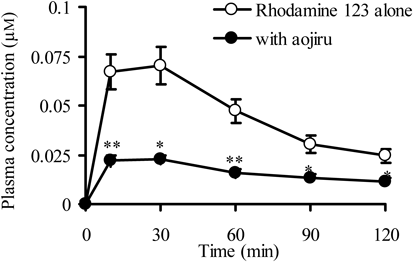
A Rho-123 solution (50 µM, 6 mL) was introduced into rat small intestinal loops with or without aojiru. Each point represents the mean±S.E. of 3–4 experiments. * p<0.05, ** p<0.01, significantly different from Rho-123 alone.
| Pharmacokinetic parameter | Rho-123 alone | Rho-123 with aojiru | p Value |
|---|---|---|---|
| Tmax (h) | 0.304±0.01 | 0.274±0.05 | n.s. |
| Cmax (µM) | 0.074±0.01 | 0.023±0.00 | p<0.01 |
| t1/2 (h) | 0.953±0.08 | 1.472±0.29 | p<0.05 |
| AUC (µmol·min/L) | 5.224±0.73 | 1.929±0.01 | p<0.05 |
Rho-123 (50 µM, 6 mL) was introduced into rat small intestinal loops with or without aojiru. Each value represents the mean with S.E. of 3–4 experiments. n.s., not significant.
Rho-123 is a well-known P-gp substrate.20) Accordingly, the result that Rho-123 absorption did not increase in the presence of aojiru may indicate the lack of inhibitory effect of aojiru on P-gp. However, some possibilities remains that aojiru stimulates the P-gp activity or induces its expression, both lead to the decreased Rho-123 absorption. It is also probable that increased Rho-123 absorption by the inhibitory effect of aojiru on P-gp was negated due to the decreased Rho-123 absorption through the binding to aojiru. Further study is required to address these possibilities, using a P-gp substrate that does not bind to aojiru.
Binding of Various Drugs to AojiruNext, the binding characteristics of seven drugs to aojiru were examined using the present centrifugation method. Figure 4 shows the results for GB and MET. Although their concentrations in the supernatant fluid were significantly lower when mixed with aojiru, the extents of the decrease in the supernatant concentration was much smaller than that observed for Rho-123 (an approximately 20% decrease for GB and less than 5% for MET). Using a dialysis method, we previously reported the weak binding of GB and negligible binding of MET to a similar type of aojiru product.16) Therefore, it could be said that the results for GB and MET obtained from the centrifugation method are in good agreement with those dialysis method.

The solutions of GB and MET were mixed with an aojiru suspension at concentrations of 12.5, 25, and 50 µM. Each column represents the mean with S.E. of 3–4 experiments. * p<0.05, ** p<0.01, *** p<0.001, significantly different from drug alone.
The results for the other five drugs are summarized in Table 2. The concentrations of DT, FEX and NFLX in the supernatant fluid were practically unchanged after mixing with aojiru, although some of the data showed statistical significance. These results indicated that these three drugs did not interact with aojiru. NFLX is known to form insoluble complexes with metallic cations such as aluminum, calcium and magnesium.21) According to the information supplied by the manufacturer, one pack (3.5 g) of the aojiru product used in this study contains 38 to 57 mg of calcium and 6.9 to 13 mg of magnesium. Thus, it was expected that, when mixed with the aojiru suspension, the NFLX concentration in the supernatant fluid would be decreased to some extent after high-speed centrifugation. However, the negligible reduction in the NFLX concentration implies that aojiru only minimally modulates the absorption of NFLX. There is a report13) showing that the features of interaction between NFLX and aojiru differ among aojiru products and that kale-based aojiru less interacts with NFLX. Our result is apparently in line with the report. As we suspended one pack of aojiru powder in 100 mL of water in this study, it is possible that the lower concentrations of calcium and magnesium failed to form insoluble complex with NFLX.
| Drugs | Conc. (µM) | Drug alone | Drug with aojiru | p Value |
|---|---|---|---|---|
| Fexofenadine | 12.5 | 11.32±0.71 | 10.80±0.04 | n.s. |
| 25 | 24.29±0.18 | 21.77±0.12 | p<0.001 | |
| 50 | 49.91±0.13 | 45.46±1.21 | p<0.05 | |
| Norfloxacin | 12.5 | 9.83±0.41 | 9.75±0.37 | n.s. |
| 25 | 18.35±0.72 | 20.10±0.57 | n.s. | |
| Diltiazem | 12.5 | 12.03±0.23 | 11.80±1.02 | n.s. |
| 25 | 23.83±0.58 | 23.36±2.58 | n.s. | |
| 50 | 47.90±1.59 | 42.86±1.56 | n.s. | |
| Imipramine | 12.5 | 10.64±0.67 | 6.55±0.32 | p<0.01 |
| 25 | 22.11±0.94 | 11.59±0.51 | p<0.001 | |
| 50 | 44.60±0.98 | 23.34±1.07 | p<0.001 | |
| Chlorpromazine | 12.5 | 12.27±0.54 | 4.21±0.05 | p<0.01 |
| 25 | 24.60±0.70 | 5.91±0.15 | p<0.001 | |
| 50 | 48.95±2.38 | 10.38±0.42 | p<0.01 |
Each value represents the mean with S.E. of 3–4 experiments. Five drugs were mixed independently with aojiru at concentrations of 12.5, 25, and 50 µM. n.s., not significant.
On the other hand, IP concentrations in the supernatant fluid decreased approximately 45%. In the case of CP, its concentrations in the supernatant fluid decreased markedly to approximately 26% of the control. According to the results of Rho-123, it is expected that, when administered together with aojiru, the intestinal absorption of IP and CP significantly decline. However, as we could not have evaluated the effect of aojiru on IP and CP by in vivo study, it is still hard to conclude that the results obtained from the centrifugation method are definitely reflected to in vivo interaction. Watanabe et al.22) previously reported that CP, IP and amitriptyline were highly adsorbed by dietary fiber, sodium alginate and glucomannan in vitro and that serum IP concentrations decreased when administered together with sodium alginate to rats. Further, Stewart and Psych23) reported that serum concentrations of doxepin, a tricyclic antidepressant, were below the therapeutic range in patients ingesting a highly fiber-rich diet and returned to normal range again when the diet was stopped. As DT (a lipophilic drug) and MET (a hydrophilic cationic drug) only slightly bound to aojiru (Fig. 4, Table 2), it is likely that the extensive binding of CP, IP and Rho-123 to aojiru depended on their cationic structure and greater lipophilicity, particularly those with a tricyclic moiety.
Reversibility of Drug Binding to AojiruTo know whether the binding of drugs to aojiru is reversible or irreversible, the release of Rho-123 and IP from aojiru was evaluated by suspending the aojiru precipitate in purified water and by centrifuging the suspension again. As shown in Fig. 5, approximately 10% of the Rho-123 and IP bound to aojiru was released at each concentration, indicating that their binding to aojiru was only slightly reversible. Therefore, a possible explanation for the extended t1/2 value after concurrent administration of Rho-123 and aojiru (Table 1) was that the Rho-123 bound to aojiru was gradually released into the intestinal lumen where it was slowly absorbed, as in the case of the intestinal absorption of a sustained-release preparation.

Aojiru precipitate was resuspended in purified water and then further centrifuged. Each column represents the mean with S.E. of 4–5 experiments.
In this study, we used an in situ loop technique to determine interactions in the intestinal absorption. Therefore, the possibility remains that the features of the pharmacokinetic interactions are somewhat different from present results when a drug and aojiru are administered together orally.
In conclusion, it was considered that the centrifugation method tested in this study was useful for predicting possible pharmacokinetic interactions between drugs and the insoluble components of aojiru, with simple and time saving experimental protocol in comparison with dialysis method. The present study also implies that the absorption of drugs, which exert similar or greater binding capacity to aojiru than Rho-123, is markedly impaired by concurrent intake of aojiru.
The authors declare no conflict of interest.