2017 Volume 40 Issue 9 Pages 1416-1422
2017 Volume 40 Issue 9 Pages 1416-1422
DW2008 is an anhydrous ethanol extract of Justicia procumbens produced by Dong-Wha Pharmaceutical, Inc., Co. as a candidate anti-asthmatic drug. In this study, DW2008 selectively reduced T helper 2 (Th2) cytokines in mouse splenocytes and ameliorated ovalbumin-induced airway inflammation by downregulating pulmonary infiltration of differential inflammatory cells and Th2 cytokines more than a decoction or ethanol extract of J. procumbens did in a mouse asthma model. DW2008 also significantly inhibited airway hyperresponsiveness and reduced the thickness of the airway epithelium. HPLC analysis showed that the major peaks (justicidin A and B) of DW2008 were higher than those of the other extracts. Justicidin A and B significantly suppressed Th2 cytokine levels in mouse spleen cells and exhibited a protective effect in ovalbumin-induced airway inflammation. Our findings indicate that DW2008 effectively inhibits allergic airway inflammatory reactions and airway hyperresponsiveness in a mouse model of asthma, suggesting its potential as an anti-asthmatic agent.
Asthma is a heterogeneous inflammatory lung disease characterized by chronic airway inflammation and bronchoconstriction. Immunoglobulin E (IgE) and various T helper 2 (Th2) cytokines, including interleukin (IL)-4 and IL-5, are commonly involved in allergic asthma. IL-4 activates B lymphocytes to produce allergen-specific IgE,1) which binds to the high-affinity IgE receptor (FcεR1) on the surface of mast cells and basophils, thereby activating them.2) Activated mast cells and basophils release various inflammatory mediators such as histamine, leukotriene, and prostaglandin D2, which induce vasodilation, contraction of the bronchial smooth muscle, and mucus secretion.2,3) Eosinophil accumulation, lung damage, and airway hyperresponsiveness (AHR) resulting from aeroallergen challenge were suppressed in IL-5-deficient C57BL/6 mice, but completely restored by reconstitution of IL-5 production with IL-5 recombinant vaccinia viruses.4) In other studies, IL-5 and eotaxin were shown as key mediators that facilitate recruitment of eosinophils in the lungs and play pivotal roles in inducing eosinophilic asthma.5,6)
Inhaled corticosteroids (ICSs) and β2-agonists have been preferentially used in asthma treatment as controllers and relievers, respectively. However, long-term use of corticosteroids may lead to several adverse effects, including bone fractures and candidiasis, while β2-agonists may exacerbate asthma.7,8) Leukotriene receptor antagonists such as montelukast (MK) and theophylline offer other controller options; however, their use is limited by their insufficient efficacy and narrow therapeutic window, respectively.
Many plants and plant-derived compounds have been shown to be effective in preventing and treating various heterogeneous diseases, such as diabetic complications and asthma, by regulating multi-targets related to inflammation or immunomodulation.9,10) Justicia procumbens (Oriental water willow) is a plant belonging to the Acanthaceae family and is widespread in Korea, India, South China, and Taiwan. A leaf decoction of J. procumbens has long been used as a folk medicine for the treatment of asthma in India,11) while its root decoction has been used against typhoid fever in Nepal.12) Furthermore, the juice of the whole plant has been used to alleviate fever, pain, and cancer in Taiwan.13) J. procumbens contains several lignans, such as taiwanin E and taiwanin E methyl ether, that exhibit anti-platelet aggregation, anti-tumor, and anti-inflammatory activities.14,15) Justicidin A (JA) has been shown to inhibit the transport of tumor necrosis factor α (TNF-α) to the cell surface in lipopolysaccharide (LPS)-activated RAW264.7 cells,16) and justicidin B (JB) isolated from Phyllanthus polyphyllus L. inhibited TNF-α and nitric oxide production in LPS/interferon (IFN)-γ-activated peritoneal macrophages.17)
However, the anti-asthmatic activities and bioactive components of J. procumbens have been not fully characterized. Thus, in the present study, we investigated the anti-allergic/anti-asthmatic effects of J. procumbens and identified the components responsible for its bioactivity.
J. procumbens was cultivated in Yong-in, Korea (GPS coordinates 37.2448 N, 127.1134 E), and authenticated by Dr. Myounghai Kwak from the National Institute of Biological Resources, Korea. A voucher specimen (NIBRVP0000469673) was deposited at the herbarium of the National Institute of Biological Resources, Korea. Dried aerial parts of J. procumbens were extracted twice with water, ethanol, or anhydrous ethanol under reflux (10-fold solvent volume). The extracts were concentrated at 60°C under vacuum, and then dried in a vacuum oven at 60°C for 12 h. Approximately 10 g water extract, 5 g ethanol extract, and 3 g anhydrous ethanol extract were obtained from 100 g J. procumbens.
Spleen Cell Culture and Cytokine AssaySpleen cells were collected from BALB/c mice under aseptic conditions. Briefly, spleens removed from 6- to 10-week-old female BALB/c mice (Orient Bio, Seongnam, Korea) were placed in phosphate-buffered saline (PBS), minced with the plunger end of a syringe, and then filtered with a 40-µm cell strainer. Following centrifugation for 5 min at 800×g, red blood cells were lysed with ammonium-chloride-potassium (ACK) lysing buffer (155 mM NH4Cl, 10 mM KHCO3, 0.1 mM ethylenediaminetetraacetic acid (EDTA), pH 7.2). After rinsing the cells twice with RPMI 1640 medium (Gibco, Grand Island, NY, U.S.A.) containing 10% fetal bovine serum, 1 mM sodium pyruvate, 0.05 mM 2-mercaptoethanol, 2 mM L-glutamine, 100 U penicillin, and 50 µg/mL streptomycin, the cells were plated at a density of 5×106 cells/mL. Viability of spleen cells was evaluated by trypan blue exclusion, which showed that over 95% of the cells were viable. Spleen cells were co-treated with 5 µg/mL concanavalin A (Con A) and the indicated drugs for 48 h. Thereafter, levels of cytokines in the supernatants of spleen cells were measured using enzyme-linked immunosorbent assay (ELISA) kits (Koma Biotech, Seoul, Korea).
Animal Model of AsthmaFemale BALB/c mice (6 weeks old upon receipt, Orient Bio) were acclimatized for 7 d. All laboratory animals were treated according to the Guide for the Care and Use of Laboratory Animals by the Institute of Laboratory Animal Resources, Commission on Life Science, National Research Council, and with the approval of the Institutional Animal Care and Use Committee of the Research Institute of Dong-Wha Pharmaceuticals (approval number: DW IACUC 14016, 14020, 15041, 16005, 16043). The mice were randomly divided into groups, and asthma groups were induced by sensitization with intraperitoneal injection of saline containing 1 mg/mL ovalbumin (OVA) and 20 mg/mL aluminum hydroxide, followed by challenge with 2 mg/mL aerosol OVA. The normal group was sensitized and challenged by saline without OVA. The OVA-sensitized mice were orally administered extracts of J. procumbens or 10 mg/kg MK (Sigma-Aldrich, St. Louis, MO, U.S.A.), or were intraperitoneally injected with 3 mg/kg dexamethasone (Sigma-Aldrich) 1 h before the OVA challenge (Fig. 1). The administered volume of all drugs was 10 mL/kg body weight. Bronchoalveolar lavage fluid (BALF) was collected by lavaging the trachea twice with 1 mL normal saline 5 h after the last challenge, and was then centrifuged at 400×g for 10 min to separate the cells. The cells were re-suspended in 0.5 mL PBS, and total cell counts were measured using a hemocytometer after staining with trypan blue. Differential cell counts were performed using the Diff-Quik staining solution kit (Sysmex, Kobe, Japan), with over 200 cells counted in images obtained from randomly selected areas. ELISA kits were used to measure levels of IL-4 (Koma Biotech, Seoul, Korea), IL-5 (R&D Systems, Minneapolis, MN, U.S.A.), IgE (Koma Biotech, Seoul, Korea), and eotaxin (Koma Biotech, Seoul, Korea).

AHR was assessed in conscious and unrestrained mice 5 h after the last aerosol challenge by whole-body plethysmography (Data Sciences International, St. Paul, MN, U.S.A.). Mice were placed in a plastic chamber and exposed to aerosolized PBS. Baseline values were measured with increasing concentrations of aerosolized methacholine solution (12.5, 25, 50 mg/mL; Sigma-Aldrich) for 1 min each, using ultrasonic nebulizers (PARI BOY SX; Pari GmbH, Starnberg, Germany). Bronchoconstriction was recorded for an additional 3 min for each concentration of methacholine aerosol. Average Penh values are expressed for each methacholine concentration as the increase over baseline Penh values.
Histological ExaminationLung tissues removed from the mice were fixed in 10% neutral buffered formalin (NBF) and then embedded in paraffin. After dehydration, the tissues were cut into 3–4-µm sections and stained with hematoxylin and eosin (H&E). The thickness was quantified blindly in a randomly selected area using an Olympus BX51-ProgRes CF microscope (Tokyo, Japan) and Image-Pro Plus 6.0 software (Media Cybernetics, Rockville, MD, U.S.A.).
HPLC AnalysisChromatographic analysis was performed on an Agilent 1200 series HPLC system (Agilent Technologies, Santa Clara, CA, U.S.A.) consisting of a pump solvent management system (G1311A), an online degasser (G1322A), an autosampler (G1329A), and a photodiode array detector (G1315D). OpenLAB chromatography software was used to collect and process the chromatographic data. Separations were performed using a reversed-phase column (Capcell Pak UG120 C18, 250×4.6 mm i.d., 5 µm; Shiseido, Tokyo, Japan). The mobile phase consisted of acetonitrile (A) and water (B). The linear gradient profile was 15% A for 5 min, 15–46% A for 35 min, 46–55% A for 20 min, 55–60% A for 10 min, 60–40% A for 5 min, and 40–15% A for 1 min. After an equilibration period of 15 min, the prepared samples (10 µL) were injected into the HPLC system. The flow rate was set at 0.8 mL/min, the temperature at 35°C, and the UV detector at 256 nm. All solutions were centrifuged at 1200–1500 rpm for 15 min, and the supernatant was used as the sample solution.
Isolation of Major ComponentsThe dried aerial parts of J. procumbens (500 g) were chopped and extracted successively twice with ethanol (3 L) under reflux. After filtration and evaporation of ethanol at reduced pressure, the ethanol extract residue (11.44 g) was suspended in water and partitioned with n-hexane (1 L×3, 5.15 g), ethyl acetate (3 L×3, 2.27 g), and n-butanol (3 L×3, 0.3 g). The n-hexane fraction was subjected to silica gel chromatography and eluted with a gradient of CHCl2–MeOH (from 100 : 0 to 0 : 100) to yield 11 fractions (JP-Hex-01–11). Fraction 4 (JP-Hex-04) was then chromatographed on a silica gel column and eluted with a gradient of CHCl2–MeOH (from 100 : 0 to 0 : 100) to yield 9 sub-fractions (JP-Hex-0401–0409).
The JP-Hex-0404 sub-fraction was separated by HPLC (Agilent 1100 series; Atlantis® Prep T3 OBD™ column, 5 µm, 150×19 mm i.d.; 70% MeOH; UV 254 nm; 10 mL/min) to obtain compound 1 (retention time (RT), 8.1 min), compound 2 (RT, 10.2 min), and compound 3 (RT, 14.3 min). The JP-Hex-06 fraction was chromatographed on a silica gel column and eluted with a gradient of n-hexane–ethyl acetate (from 10 : 1 to 1 : 1) to yield 19 sub-fractions (JP-Hex-0601–0619). The JP-Hex-0606 sub-fraction was separated with HPLC (Agilent 1100 series; Atlantis® Prep T3 OBD™ column, 5 µm, 150 mm×19 mm i.d.; 70% MeOH; UV 254 nm; 10 mL/min) to obtain compound 4 (RT, 14.7 min). The peaks for these compounds were identified in the HPLC profile of J. procumbens by comparison of retention time and UV spectrum. Purity of these compounds in HPLC was checked, and single compounds (compounds 1–4) with purity over 95% were obtained.
Statistical AnalysisAll data were statistically analyzed using SPSS for Windows (PASW Statistics 18.0, professional pack, SPSS). The results are expressed as means±standard error (S.E.). Variance homogeneity was evaluated using Levene’s test. Data were analyzed by one-way ANOVA, followed by a Dunnett’s test if Levene’s test indicated no significant deviations from variance homogeneity. A nonparametric Kruskal–Wallis H comparison was conducted in case of significant deviations from variance homogeneity. When the Kruskal–Wallis H-test indicated a significant difference, a Mann–Whitney U-Wilcoxon rank-sum W test was performed to determine the specific pairs that were significantly different.
We analyzed the extracts of J. procumbens by HPLC, and the major peaks of J. procumbens at RT 45–55 min in the HPLC analysis (256-nm wavelength) were determined (Fig. 2). The peaks of JP-AE were approximately 1.5-fold higher than those of JP-E, while the peaks of JP-D were hardly detectable (Fig. 2A). We isolated four individual compounds from the extract of dried J. procumbens by column chromatography. These compounds were identified as justicidin B (JB, 1), justicidin A (JA, 2), justicidin C (JC, 3), and phyllamyricin C (PC, 4) by NMR spectroscopy (Fig. 2B). The NMR spectra of these compounds were in accordance with previously reported data.18–20) The peaks of these compounds were identified in the HPLC profile of J. procumbens by comparing retention time and UV spectrum: 1, RT 46.65 min; 2, RT 49.99 min; 3, RT 53.81 min; 4, RT 53.81 min. The contents of JA and JB in JP-AE were calculated using standard compounds with purity over 95% by HPLC. JP-AE contained approximately 46 mg/g JA and 33 mg/g JB.

(A) HPLC analysis of Justicia procumbens. (B) Structures of the major components of anhydrous ethanol extract of J. procumbens (JP-AE). 1: justicidin B (JB), 2: justicidin A (JA), 3: justicidin C (JC), 4: phyllamyricin C (PC).
We investigated the inhibitory effects of J. procumbens on levels of Th1/Th2 cytokines stimulated with Con A, a T cell activator, in mouse spleen cells. None of the extracts affected Con A-induced release of Th1 cytokines, IL-2, and IFN-γ. Although JP-D and JP-E slightly augmented the release of IFN-γ, the increase was not significant (Fig. 3A). In contrast, both JP-E and JP-AE selectively inhibited Con A-induced release of IL-4 and IL-5 Th2 cytokines; JP-D was less potent in reducing IL-5 levels than JP-E and JP-AE and did not significantly affect IL-4 levels (Fig. 3B). However, the treatment with 0.5 µM dexamethasone markedly reduced both Th1 and Th2 cytokines to basal values. (Figs. 3A, B).
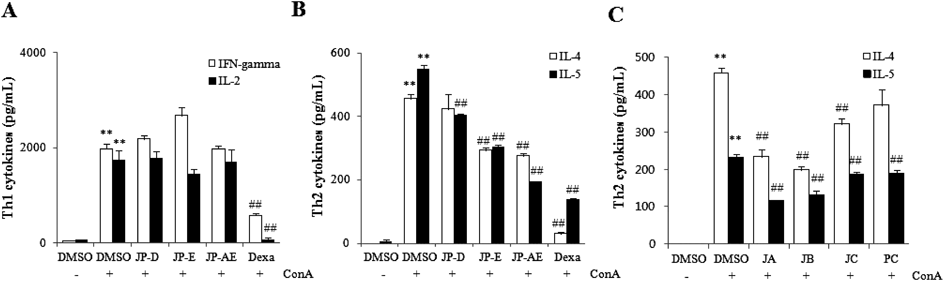
(A and B) BALB/c mouse spleen cells were co-treated with 5 µg/mL concanavalin A (Con A) and 2 µg/mL of the indicated extracts or 0.5 µM dexamethasone (Dexa, A and B) and 0.5 µM justicidin A (JA), 0.5 µM justicidin B (JB), 5 µM justicidin C (JC), or 5 µM phyllamyricin C (PC) (C), for 48 h. Supernatants of spleen cells were collected and Th cytokines levels were measured using an ELISA kit. All data are expressed as means±S.E. (n=3). ** p<0.01 vs. DMSO, ## p<0.01 vs. Con A. JP-AE, anhydrous ethanol extract of J. procumbens; JP-D, decoction of J. procumbens; JP-E, ethanol extract of J. procumbens.
As shown in Fig. 3C, treatment of spleen cells with 0.5 µM JA and JB reduced IL-4 levels by 49 and 57%, respectively, and IL-5 levels by 50 and 43%, respectively. Although JC and PC also inhibited Con A-induced release of IL-4/5 cytokines, their inhibitory effects were weaker than those of JA and JB, and they only exerted their inhibitory effects at a higher concentration (5 µM).
Anti-allergic Airway Inflammatory Effects of J. procumbens Extracts in an OVA-Induced Mouse Model of AsthmaTo confirm the alcoholic extracts (JP-E and JP-AE) were more efficacious for treating allergic responses than the water extract (JP-D) in vitro, a mouse model of asthma was established by sensitizing and challenging mice with OVA (Fig. 1).
In the OVA-immunized group, the number of differential cells, BALF IgE levels, and BALF Th2 cytokine levels were markedly higher than those in the saline-treated (normal) group (Fig. 4). Similar to the earlier cellular results, JP-AE substantially inhibited the infiltration of total inflammatory cells and eosinophils into BALF (Fig. 4A) and reduced the levels of BALF IgE by 91.7% (Fig. 4B), BALF IL-4 by 39.2% (Fig. 4C), BALF IL-5 by 51.7% (Fig. 4D), and BALF eotaxin by 66.5% (Fig. 4E). JP-D and JP-E also inhibited infiltration of inflammatory cells and reduced the levels of BALF IgE, IL-4, IL-5 and eotaxin; however, their effects were weaker than those of JP-AE. Based on these results, we developed a candidate anti-asthmatic drug, labeled DW2008 by the Dong-Wha Pharmaceutical Company, via further optimization of JP-AE.

BALB/c mice sensitized and challenged with ovalbumin (OVA) were orally administered 200 mg/kg body weight of the indicated extracts. (A) Differential cell counts, (B) bronchoalveolar lavage fluid (BALF) IgE levels, (C) BALF interleukin (IL)-4 levels, (D) BALF IL-5 levels, and (E) BALF eotaxin levels are shown. All data are expressed as means±S.E. (n=5). ** p<0.01 vs. normal group, # p<0.05 vs. OVA group, ## p<0.01 vs. OVA group. JP-AE, anhydrous ethanol extract of J. procumbens; JP-D, decoction of J. procumbens; JP-E, ethanol extract of J. procumbens.
To confirm the anti-allergic airway inflammatory effect of DW2008, we conducted H&E staining of lung sections. As shown in Fig. 5, infiltration of inflammatory cells into the lung of OVA-exposed mice was significantly higher than that of normal mice and was restored in OVA-sensitized mice treated with DW2008 (Fig. 5A). Epithelial and airway smooth muscle thickness was also significantly reduced in the OVA group treated with DW2008 (Fig. 5B).

BALB/c mice sensitized and challenged with ovalbumin (OVA) were orally administered 200 mg/kg body weight DW2008. (A) H&E staining and (B) subepithelium smooth muscle thickness and epithelium thickness (µm) are shown. All data are expressed as means±S.E. (n=5). * p<0.05 vs. normal group, # p<0.05 vs. OVA group. JP-AE, anhydrous ethanol extract of Justicia procumbens.
Broncho-constriction was induced by methacholine aerosol in OVA-induced asthmatic mice and evaluated by calculating Penh values (Fig. 6). Penh values in the OVA group were significantly higher than those in the normal group. In addition, Penh values significantly decreased in the OVA group treated with DW2008 and were similar to those observed in the OVA group treated with dexamethasone.

BALB/c mice sensitized and challenged with ovalbumin (OVA) were exposed to increasing concentrations of methacholine (12.5–50 mg/mL). The anhydrous ethanol extract of J. procumbens (JP-AE, 200 mg/kg) was orally administered and dexamethasone (3 mg/kg) was intraperitoneally injected 1 h before OVA challenge for 10 d. Values are expressed as means±S.E. (n=6). ** p<0.01 vs. normal group, # p<0.05 vs. OVA group.
Administration of 50 mg/kg ca. 200 mg/kg body weight DW2008 resulted in similar anti-asthmatic effects through further experimentation (data not shown). To determine the active compounds of DW2008, asthmatic BALB/c mice induced by OVA were orally administered 10 mg/kg body weight JA, JB, MK, or 50 mg/kg DW2008 (Fig. 7). JA administration decreased infiltration of inflammatory cells and BALF IgE levels less than or equal to those observed for MK administration; however, it reduced BALF IL4/5 and eotaxin levels more than MK did. JB reduced the infiltration of total inflammatory cells and eosinophils, BALF IgE levels, IL 4/5, and eotaxin levels more than or equal to that of MK. Although DW2008 contains less JA (approximately 2.3 mg/kg) and JB (approximately 1.7 mg/kg) than that administered to the JA and JB single-treatment groups (10 mg/kg body weight), DW2008 treatment resulted in equivalent or further inhibition of all allergic airway inflammatory biomarkers tested in this study. In addition, the inhibitory effects of DW2008 were superior to those of MK for all biomarkers tested in this study.

BALB/c mice sensitized and challenged with OVA were orally administered justicidin A (JA, 10 mg/kg), justicidin B (JB, 10 mg/kg), montelukast (MK, 10 mg/kg), or DW2008 (50 mg/kg). (A) Differential cell counts, (B) bronchoalveolar lavage fluid (BALF) IgE levels, (C) BALF interleukin (IL)-4 levels, and (D) BALF IL-5 levels are shown. All data are expressed as means±S.E. (n=5). ** p<0.01 vs. normal group, #p<0.05 vs. OVA group, ##p<0.01 vs. OVA group.
In this study, we demonstrated that the anti-allergic airway inflammatory effects of J. procumbens depend on selective regulation of Th2 cytokines and identified its main active components. We hypothesized that J. procumbens exerts anti-allergic asthma effects because its decoction has long been used as a folk medicine for the treatment of asthma.11) In our study, alcohol extracts of J. procumbens selectively inhibited Con A-induced release of the Th2 cytokines, IL-4 and IL-5, in spleen cells. The findings suggest that alcohol extracts of J. procumbens may regulate Th2 cell-driven allergic responses. These selective Th2 inhibitory effects may be associated with regulation of the T cell Ig and immunoreceptor tyrosine-based inhibitory motif (ITIM) domain receptor (TIGIT). A recent study demonstrated that TIGIT enhances Th2 immunity, and its inhibition has no effect on either Th1 or Th17 polarization; in vivo blockade of TIGIT using monoclonal antibodies suppressed hallmarks of allergic airway diseases.21) Hence, alcohol extracts of J. procumbens may regulate TIGIT. However, further studies on their mechanism of action and identification of targets related to the regulation of selective Th2 cell responses are necessary.
In our experimental mouse model of asthma, the anti-allergic airway inflammatory effects of alcohol extracts were superior to those of the water extract (decoction). Furthermore, JP-AE, extracted using anhydrous ethanol (>99.5% ethanol content), exerted stronger anti-allergic effects than JP-E extracted with ethanol (95.1–96.9% ethanol content). This result correlates with the higher concentration of active components, such as JA and JB, in JP-AE. The HPLC peak areas of the active components in JP-AE were more than 1.5-fold greater than those in the active components of JP-E. These results suggest that the content of JA and JB determines the anti-allergic asthma efficacy of J. procumbens, and that JP-AE may have a therapeutic role in ameliorating allergic asthma. Therefore, DW2008 was developed from JP-AE through further optimization. Although the overall concentrations of JA and JB administered to the DW2008 treatment groups were lower than those administered to the JA and JB single-treatment groups, DW2008 treatment ameliorated allergic airway inflammation in the OVA asthma model to the same extent as that observed in the JA and JB single-treatment groups. These results imply that a combination of JA and JB may synergistically exert anti-allergic airway inflammatory effects, or that unknown components of DW2008 augment the effects of JA and JB. These components should be identified through further analysis. Pre-clinical toxicity testing is currently on-going according to Good Laboratory Practice (GLP) guidelines before clinical testing can be conducted for development of DW2008 as an anti-asthma drug in Korea.
This research was supported by the Korea Drug Development Fund, which is funded by the Ministry of Science, Information and Communication Technology, and Future Planning, the Ministry of Trade, Industry, and Energy, and the Ministry of Health and Welfare (KDDF-201512-03, Republic of Korea).
All authors are employees of Dong-wha Pharmaceutical Company.