2017 Volume 40 Issue 9 Pages 1439-1446
2017 Volume 40 Issue 9 Pages 1439-1446
Short chain fatty acids acetate and propionate have been demonstrated protective function in the intestinal mucosa. However, their impact on gastric mucosa has not yet been elucidated. The current study aimed to investigate the potential protective effects of acetate and propionate against ethanol-induced gastric mucosal lesion and the underlying mechanism in mice. ICR mice were orally treated with acetate and propionate, respectively, 30 min prior to the establishment of gastric mucosal injury model by challenge with absolute ethanol. The gastric samples were collected for the detection of oxidative, inflammatory and apoptotic related parameters. Acetate, but not propionate, attenuated the severity of gastric mucosal damage as evidenced by the gross changes of gastric mucosa, pathological aberrations. Acetate alleviated oxidative stress as shown by the increase in glutathione (GSH) content and superoxide dismutase (SOD) activities, and the decrease of malondialdehyde (MDA) level. The elevated concentrations of interleukin (IL)-1β, tumor necrosis factor (TNF)-α and IL-6, and the activation of nuclear factor-kappaB (NF-κB) p65 by ethanol stimulation was also reduced by acetate. Moreover, the anti-inflammatory factors, IL-4, LXA4 and IL-10, were up-regulated in acetate treated group. With respect to gastric mucosal apoptosis, acetate suppressed caspase-3 activity and BAX expression in favor of cell survival. These favorable actions were maybe associated with up-regulation of the gastric MUC5AC, the key defense factor of gastric mucosal system. These findings accentuate the gastroprotective actions of acetate in ethanol-induced gastric injury which were mediated via concerted multi-prolonged actions, including suppression of gastric oxidation, inflammation and apoptosis and promotion of MUC5AC expression.
Gastric mucosal injury is the main type of inflammation related diseases of the stomach.1,2) Helicobacter pylori infection and irregular eating habits mainly attributed to chronic gastric ulcer.3) However, the excessive of alcohol comsumption4) and high dose of nonsteroidal anti-inflammatory drugs (NSAIDs)5) are the important factors causing acute gastric mucosal lesion. Ethanol can be directly absorbed and weaken gastrointenstinal mucosa.6,7) Although several orthodox drugs clinically used for treatment of this disease are effective, they cannot decrease with the high morbility of ethanol-induced gastric mucosal lesion. Therefore, it is necessary to find other alternative therapies to solve the problem.8)
Oxidative stress9) and inflammation4) are the main mechanisms of ethanol-induced gastric mucosal lesion. It is well proved that interleukin (IL)-1β,10) tumor necrosis factor (TNF)-α11) and IL-612) are the most important cytokines to deteriorate the inflammatory injury, which regulated by nuclear factor-kappaB (NF-κB) p65. Moreover, inflammation and oxidation can be interactive to induce apoptosis, which plays a crucial role in the mechanism of gastric ulceration.13) A weakened defense of gastric mucosa is also a critical factor for ethanol-induced gastric mucosal lesion. MUC5AC is an important parameter to reflect the gastric defense level.14,15) However, the current therapies in clinic are short of reagents to enhance mucosal barrier of stomach.16)
Short chain fatty acids (SCFAs) such as acetate, propionate and butyrate, are the metabolites of anaerobic bacteria, which are not only produced in intestinal tract but also present in fermented foods, and even are used as food additives. Recent studies showed that SCFAs play a crucial role regulating immune reaction,17) inflammation18,19) and even neural function.20) In series of studies, we are focused on the SCFAs effects on ethanol-induced gastric mucosal lesion. We originally reported that probiotic Clostridium butyricum21) and its metabolite, butyrate,22) can protect gastric mucosa from lesion. To date, the most extensively studied and best documented functions of acetate and propionate are their effect on inflammatory bowel diseases (IBDs).23) The current study aims to investigate whether acetate or propionate can ameliorate gastric mucosal lesion.
We employed ethanol-induced acute gastric lesion model in mice to detect the pathological changes of gastric mucosa, the oxidative, inflammatory and apoptotic parameters, and MUC5AC level to evaluate the effect of acetate or propionate on gastric mucosa and the underlying mechanism.
Male ICR mice purchased from Experiment Animal Center of Wenzhou Medical University (Wenzhou, China) were kept in specific pathogen free (SPF) animal quarters under a 12 h light–12 h dark cycle. The mice were allowed standard chow and tap water ad libitum. All the animal experimental procedures in the present study were in accordance with the guidelines of the Animal Ethics Committee of Wenzhou Medical University.
Fifty four mice were randomly divided into 9 groups, including normal control group, model group, Omeprazole group and six groups treated with low, middle and high dosages of acetate or propionate (n=6/group). Equal volume and pH of NaHCO3 solution was orally given to the mice of model group via an orogastric tube 30 min prior to modeling. Mice in Omeprazole group were intraperitoneally treated with Omeprazole by dose of 20 mg/kg.
Acetate and Propionate TreatmentAnimals treated with acetate or propoinate were orally administrated by low dose (1 g/kg), middle dose (2 g/kg) and high dose (4 g/kg), respectively, 30 min prior to model establishment.
Gastric Mucosal Lesion Model Induced by EthanolFasted-mice were starved for 24 h by removing only food (water was provided ad libitum). Absolute ethanol 0.01 mL/g was orally administrated to mice except those of the normal control group for the model establishment. One hour later, the mice were sacrificed to collect gastric tissue samples.
Gastric Mucosal Lesion DeterminationImages of stomachs were taken by a digital camera, then analyzed by the Image-Pro Plus (IPP) 6.0 software to calculate the ratio of hemorrhagic lesion area over total gastric mucosa. The percent area of gastric ulcer in different groups was preliminarily analyzed to evaluate the effects of acetate and propionate.
Histopathological Observation of Gastric TissuesThe specimens of stomachs were fixed with 4% paraformaldehyde and then sectioned at a thickness of 5 µm for hematoxylin and eosin (HE) staining to observe histopathological changes under a light microscope.
All the histopathology specimens were scored by the gastric mucosal injury and neutrophils infiltration under light microscopy as previously described.22) A scale of 0–4 was used.
Caspase 3 Activity Assay in Gastric TissueFrozen gastric tissues were homogenized at 4°C in chilled lysis buffer and centrifuged at 15000×g for 30 min at 4°C. Caspase 3 activity was assessed on the supernatants in accordance with the manufacturer’s instructions.
Detection of Oxidative Stress IndicatorsThe malondi-aldehyde (MDA) and glutathione (GSH) content, and total superoxide dismutase (SOD) activities in gastric tissues were determined using the methods of thiobarbituric acid,24) 5,5′-dithio-bis(2-nitrobenzoic) acid (DTBN)25) and xanthine oxidase,26) repsectively. Absorbance was detected using a 722N spectrophotometer (the Scientific Instrument Co., Ltd., Shanghai, China). Procedures were done following the instructions provided by each kit.
Examination of Cytokines in Gastric TissuesLevels of IL-1β, TNF-α, IL-6, IL-10, IL-4, LXA4 and MUC5AC in gastric tissues were tested using commercial available enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer’s instructions. Briefly, gastric tissue homogenate was pippetted into a 96-well plate coated with the primary antibodies, and incubated for 3 h. Then, biotin conjugate was added for an incubation of 45 min at room temperature after twice washing. After washing non-specific binding, streptavidin-horseradish peroxidase (HRP) was incubated for 45 min. Then Chromogen was pippetted into each well for the absorbance measurement at 450 nm. The levels of IL-1β, TNF-α, IL-6, IL-10, IL-4 and LXA4 were calculated by standard curves according to ELISA kits instructions.
Measurement of Gastric Wall Mucus (GWM)GWM was measured by an improved procedure reported.27) Briefly, the glandular segments of gastric tissues were collected and weighed for incubation in 1% Alcian blue buffer (pH 5.0). After being rinsed to remove the remnant dye, the combined dye was extracted from the gastric wall mucus with 4 mL magnesium chloride solution. Then an equal volume of diethyl ether was added to the samples. After routinely centrifuge, the aqueous layer was collected for reading of absorbance at 580 nm.
Quantitative (Q) PCR Analysis for MUC5AC and Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH)Total RNA was isolated from cultured cells by Trizol reagent from ComWin Biotech, Co. (Beijing, China) according to the manufacturer’s instruction. The mRNAs for MUC5AC and GAPDH were quantified by real-time PCR using the corresponding primers and the QuantiTect SYBR Green PCR kit from Applied Biosystems, Co., Ltd. (Foster City, CA, U.S.A.).
ImmunohistochemistryPrimary antibodies for BAX was added to the paraffin sections for immunohistochemistry (IHC) analysis at a dilution of 1 : 200. After the routine antibody incubation, diaminobenzidine (DAB) was used as the chromogen to visualize the positive signals.
Western BlottingThe gastric tissue samples were quantified using the bicinchoninic acid (BCA) assay, followed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The separated proteins were then transferred to a polyvinylidene difluoride (PVDF) membrane. Western blot analysis was performed with a specific primary antibody (1 : 1000) against phosphorylated, total p65 and BAX. After conventional antibody incubation, the protein band of interest was visualized by enhanced chemiluminescence (ECL).
Statistical AnalysisThe data from different group were presented as mean±standard deviation (S.D.) Significant differences were determined by the Bonferroni test. A p value less than 0.05 was considered statistical significant.
To evaluate the effects of acetate and propionate on ethanol-induced gastric mucosal lesion, we analyzed macroscopic lesions of gastric mucosa. The data demonstrated that extensive hemorrhagic injuries induced by ethanol challenge (Fig. 1A). We found that acetate, but not propionate, in a dose-dependent manner attenuated the mucosal lesions (Fig. 1A). The protective effect of the high dose of acetate was even better than Omeprazole positive control (Fig. 1A). Consistent with the result of macroscopic observation, the quantitative analysis of the ulcer areas also showed that the gastric mucosal protection of acetate was in a dose-dependent style (Fig. 1B).

(A) The gross morphological changes of the gastric mucosa of mice (scale bar=5 mm). (B) Statistical analysis on the ethanol-induced gastric mucosal ulcer area. Fifty four mice were evenly divided into 9 groups. The mice were fasten for 24 h, then were orally treated with different doses of acetate, propionate or with Omeprazole (20 mg/kg) 30 min before establishing the model by ethanol (0.01 mL/g). The gross morphological changes of the gastric mucosa were evaluated after the euthanasia of mice. N: normal control group; M: model group; At1–At3 represent the groups treated with acetate by the dose of 1, 2 and 4 g/kg, respectively; Pt1–Pt3 represent the groups treated with propionate by the dose of 1, 2 and 4 g/kg, respectively; O: Omeprazole group. The data are expressed as the means±S.D. (n=6). *: p<0.05, **: p<0.01, compared to the model group; ##: p<0.01 compared to the Omeprazole group.
Histological examination was employed to further estimate the effects of acetate on gastric mucosal lesion (Fig. 2). When compared with the normal control group, there were extensive injuries to the gastric mucosa of model group, such as necrotic damage of mucosa, leukocyte infiltration and even local detachment of the gastric mucosa. However, acetate dose-dependently attenuated the gastric mucosal damages.

The gastric tissue sections (n=6/group) were routinely dehydrated, and then stained with hematoxylin and eosin (HE) followed by observation with a microscope (×20, scale bar=100 µm). N: normal control group; M: model group; At1–At3 represent the groups treated with acetate by the dose of 1, 2 and 4 g/kg, respectively; O: Omeprazole group.
Following the results of the dose-dependent gastric mucosal protection of acetate described above, we chose high dose of acetate to experimentally investigate the underlying mechanism. Since oxidative stress is a critical factor in pathogenesis of gastric mucosal lesion, we tested the activity of SOD and the levels of GSH and MDA to reflect the oxidative damage and evaluate the anti-oxidation of acetate. The results showed that ethanol challenge as expected increased the level of MDA, and reduced the GSH content and SOD activity, however, acetate significantly perfected the oxidative changes (p<0.01, Fig. 3).

The gastric tissue samples were homogenized to detect MDA, GSH and SOD, according to the corresponding instructions of kits. The concentrations of these detected oxidative parameters in the gastric tissue were normalized by protein quantity using BCA assay. The data were expressed as the means±S.D. (n=6). N: normal control group; M: model group; At3: the group treated with acetate by the dose of 4 g/kg. ∆∆ p<0.01, compared with the normal control group; **: p<0.01, compared with the model group.
Because of the importance of the overwhelming inflammation in gastric mucosal lesion, we measured the levels of IL-1β, TNF-α and IL-6, which are representative proinflammatory cytokines, in the gastric tissues by ELISA assays. In contrast to the normal control group, the concentrations of all three proinflammatory factors were significantly higher in the ethanol-challenged mice. Acetate dramatically attenuated the ethanol-induced elevation of these proinflammatory cytokines (p<0.01, Fig. 4A). Furthermore, Western blotting showed that NF-κB p65, the key modulator of signaling pathways to produce inflammatory cytokines during gastric mucosal lesion,28) was significantly phosphoralated after ethanol stimulation, and was inhibited by acetate (Fig. 4B).
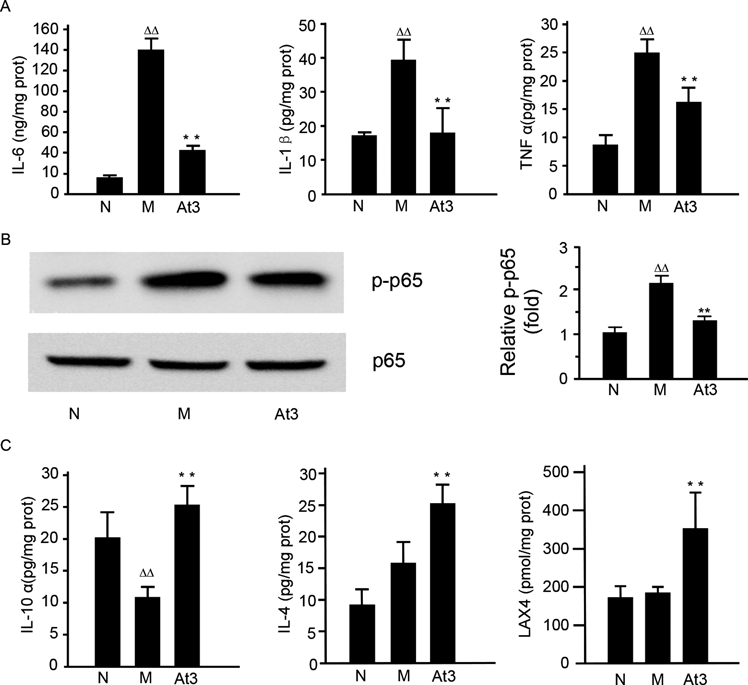
(A) The level of IL-1β, TNF-α and IL-6 in the gastric tissues. (B) NF-κB p65 activation in the gastric tissues. (C) The level of IL-10, IL-4 and LXA4 in the gastric tissues. Gastric tissue homogenate was prepared for cytokines determination by ELISA and Western blot analysis of NF-κB p65 activation. N: normal control group; M: model group; At3: the group treated with acetate by the dose of 4 g/kg acetate. The data were expressed as the means±S.D. (n=6). ∆∆ p<0.01, compared with the normal control group; ** p<0.01, compared with the model group.
Moreover, we also detected IL-10, IL-4 and LXA4, typical anti-inflammatory cytokines, to further understand the mechanism of anti-inflammation for acetate. The results showed that IL-10, IL-4 and LXA4 were obviously up-regulated by acetate (p<0.01, Fig. 4C).
Acetate Down-Regulated BAX and Caspase 3 in the Gastric Mucosa of MiceEthanol instillation instigated apoptosis in inflamed mucosa as shown by the significant increase of the express and activity caspase 3, a reliable indicator for apoptosis and the elevation of BAX protein (Fig. 5). However, acetate counteracted these changes in favor of cell survival, implicating suppression of apoptosis as a crucial event in aceate protection against ethanol-induced gastric injury.

(A, B) The IHC staining for BAX. The gastric samples were fixed and sectioned for staining by the primary antibody of BAX. The brown granules in cells were positively considered. A: ×20, scale bar=100 µm; B: ×40, scale bar=50 µm. (C) The activity of caspase 3 analysis. (D) The Western blot detection for BAX and caspase 3. The gastric tissue homogenate was prepared for determination of caspase 3 activity and Western blot. N: normal control group; M: model group; At3: the group treated with acetate by the dose of 4 g/kg acetate. The data were expressed as the means±S.D. (n=6). ∆∆ p<0.01, compared with the normal control group; ** p<0.01, compared with the model group.
As SCFAs are well understood to repair intestinal mucus, we performed quantitative assay of gastric wall mucus to further observe the GWM changes. The results showed that GWM was severely damaged by ethanol stimulation, however, this deteriorated process was significantly reversed by acetate (p<0.01) (Fig. 6). Furthermore, we also employed the ELISA and QPCR to detect the MUC5AC, namely gastric mucin, which plays the critical role in gastric mucosal defense system. The data showed that MUC5AC was severely reduced by ethanol stimulation, whereas acetate significantly increased its content (p<0.01, Fig. 6).

(A) gastric wall mucus content (GWM). The mucosa part of gastric wall was obtained from the gastric samples for the staining with alcian blue by a routine method to calculate the GWM (B) The ELISA detection for MUC5AC. The gastric tissue homogenate was prepared for determination of MUC5AC. The level of MUC5AC in the gastric tissues was normalized by the protein quantity assay. (C)The QPCR analysis for MUC5AC. The total RNA in gastric tissues was extracted for detection of MUC5AC and GAPDH mRNA by QPCR. The targeted mRNAs in normal control group was calibrated as 1 to analyze the content of MUC5AC in the gastric tissues. N: normal control group; M: model group; At3: the group treated with acetate by the dose of 4 g/kg acetate. The data were expressed as the means±S.D. (n=6). ∆∆ p<0.01, compared with the normal control group; ** p<0.01, compared with the model group.
Although SCFAs, acetate and propionate, have been used as nutrition adjust agents for a few of decades, we know little about the biological effects of them on the body. As the metabolites of intestinal microbiota, SCAFs at high concetration and beneficial to intestinal mucosa,29,30) but we hypothesized that gastric mucosa should be responsive to acetate and propionate, since stomach with the similar histological structure, as an extension of intestine, should be sensitive to the necessary nutrition for digestive pipe. While performing experiments to test the intestine-like protection of them for gastric mucosa, we only discovered that acetate, but not propionate, protected gastric mucosa against lesion. Although the effective dose of acetate is higher than other chemicals, protective function of acetate gastric mucosa is potentially used in human, because acetate is a food adjust agent with a high LD50 (6.891 g/kg, mouse, oral).
Due to the lack of effective therapies to promote the repairment of gastric mucosa and the damaged gastric mucosal defense, the prevention and treatment of alcohol-induced gastric mucosal lesion currently remains a big problem to be solved.31) We and other teams are trying to find workable strategies to positively promote the mucosal defense system of stomach.21,32) In this study we developed acute gastric mucosal lesion by ethanol which can directly damage the mucosal layer, and then secondarily induce lesion by digestive gastric juice. Thus, this classical model of gastric mucosal lesion is suitable for screening chemicals with gastric mucosal protective capacity. Acetate is not the acid inhibitor, but pH of its solution is alkaline. Thus, we used the same pH value of NaHCO3 as control and found the gastric mucosal protection of acetate is not pH dependent. Therefore, it indicated that acetate may directly promote the gastric mucosal defense against the lesions induced by ethanol. This result was further supported by the finding that the GWM content and MUC5AC expression were significantly up-regulated by acetate. Consistent with the results obtained in previous study on IBDs, acetate prevented mucosal damage due to inflammatory injury,23) suggesting that acetate exerted its protective effect on gastric mucosal lesion by enhancing mucosal defense mechanism, rather than inhibiting of acid secretion.
Over-excessive inflammation is the critical factor contributing to ethanol-induced gastric mucosal lesion. However, the current therapies in clinic are short in effective drugs to eliminate inflammation. Oppositely, NSAIDs, which are extensively used in clinic to anti-inflammation by inhibiting cyclooxygenase (COX) to block the production of prostaglandins (PGs), aggrevate the local inflammation of stomach,33) because of the critically protective role of PGs for gastric mucosa. In this experiment, we demonstrated that acetate obviously reduced the inflammation of gastric mucosa by showing a decrease of IL-1β, TNF-α and IL-6. Furthermore, we also showed that NF-κB p65, the key regulator of proinflammatory cytokines, was inhibited by acetate, which is consistently with Soliman ML.34,35) Moreover, we found that anti-inflammation effect of acetate is via up-regulation of IL-10, IL-4 and LXA4. In fact, it has been reported that the anti-inflammation effect of acetate is via up-regulation of IL-10 and IL-435) by other researchers. However, the significant increase of LXA4 induced by acetate, which has been shown to be protective for gastric mucosa,36,37) was observed for the first time in our study. Therefore, the anti-inflammation effect of acetate may be via different pathway. We will further investigate the underlying mechanism in the next step.
Due to the tissue damage ability, reactive oxygen species (ROS)7,38) play an important role in the pathogenesis of ethanol-induced gastric mucosal lesion. Our data showed that SOD activity and GSH content were reduced but the MDA level was increased after ethanol stimulation, indicating that the oxidative stress disturbance deteriorated the lesion of gastric mucosa. Whereas acetate significantly attenuated the oxidative state as described in Results. Previous researches showed that acetate obviously inhibited the activation of neutrophils which can produce amount of ROS.39–41) Therefore, anti-oxidation function of acetate may be attributable to the inhibition of neutrophils.
The data presented in this study showed that acetate significantly attenuated the caspase 3 activity and BAX expression, indicating an anti-apoptotic effect for acetate. Apoptosis can be induced by the excess of inflammation and oxidation, and contributes to the damage of gastric mucosa. Thus, apoptosis suppression by acetate maybe attributed to its anti-inflammation and anti-oxidation effects in accordance with our results and analysis. However, the underlying mechanism of anti-inflammation and anti-oxidation for acetate against gastric mucosal lesion is not completely elucidated in the present study. Acetate has been reported to activate its special receptor, GRP43, to inhibit inflammation.1,2) Otherwise, acetate has been also documented to regulate gene expression to inhibit inflammation as a histone deacetyltranferase (HDAC) inhibitor.42) Since acetate was shown to promote the expression of MUC5AC, we presumably speculated that acetate reinforced the mucosal defense to attenuate inflammation and oxidation via GRP43 or HDAC inhibition. In the next step, we plan to investigate further this mechanism.
In summary, we originally demonstrated that SCFA acetate, a common and simple compound as well as a food additive used in food industry, can protect gastric mucosa from injury induced by ethanol via suppression of gastric oxidation, inflammation and apoptosis and promotion of MUC5AC expression.
This study was supported by the Technology Bureau of Wenzhou (No. y20150009, y20130215), the Project of Science and Technology Department of Zhejiang Province (No. 2017C33068). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declare no conflict of interest.