2017 Volume 40 Issue 9 Pages 1586-1589
2017 Volume 40 Issue 9 Pages 1586-1589
Meibomian gland dysfunction (MGD) is the leading cause of dry eye, and although it affects approximately 4% of the population, treatment options remain limited. Topical azithromycin is one of the most promising pharmacological agents because of its multiple mechanisms of action and long sustainability. Azithromycin is frequently used as an off-label medication in the U.S. However, although azithromycin is presumed to act directly on meibomian gland cells, the mechanisms of action that contribute to its clinical efficacy remain unclear because no studies using a pharmacokinetic approach have been performed. Therefore, we aimed to clarify whether topical azithromycin reaches the meibomian glands sufficiently to generate a biological effect. We measured azithromycin concentrations in rabbit meibomian glands collected using a recently developed method. Moreover, we also visualized the azithromycin micro-distribution using desorption electrospray ionization (DESI) imaging. Azithromycin concentration in the meibomian glands reached only 0.8 µg/g tissue following a single application of a 1% azithromycin ophthalmic solution and was 1000-fold lower than the concentration in conjunctival epithelium. Similarly, no signal was observed in the meibomian glands on DESI images. Our results clearly demonstrated that topical azithromycin had limited access to the meibomian glands and was predominantly distributed in ocular surface tissues such as the palpebral conjunctiva and lid margins. These findings provide new insight into the clinical responses to topical azithromycin therapy and will aid in the further development of effective drugs with more suitable pharmacokinetic properties.
Meibomian glands are sebaceous glands embedded within specific types of dense connective tissue in the eyelid. They are primarily responsible for producing meibomian oil (meibum), which plays a physiologically important role in promoting tear film stability and preventing aqueous tear evaporation on the ocular surface.1) Meibomian gland blockages are clinically defined as meibomian gland dysfunction (MGD), which is the leading cause of dry eye disease. However, there is no pharmacologically approved drug useful in treating MGD. Azithromycin ophthalmic solution (AzaSite®; InSite Vision, a SUN PHARMA company, Alameda, CA, U.S.A.) is one of the most promising candidates for MGD treatment.2) Although it has been approved only for the treatment of bacterial conjunctivitis, off-label application of topical azithromycin is the most commonly used pharmaceutical treatment in the U.S. for the management of MGD.3) In addition to its broad anti-bacterial spectrum, azithromycin inhibits pro-inflammatory cytokines and restores lipid properties. Indeed, several clinical studies have demonstrated that topical azithromycin ophthalmic solution significantly relieved the clinical signs and symptoms of MGD.4–6) Although significant progress has been made in human clinical studies to better understand the mechanism of MGD, fundamental pharmaceutical research still lags behind. Most recently, several animal models have been established based on MGD pathogenesis or specific signs.7–10) Nevertheless, a pharmacokinetic approach is entirely lacking due to its unique location and the technical hurdles of collecting tissue samples. Therefore, azithromycin distribution, especially in meibomian glands, stimulates an intriguing discussion in terms of the pharmacodynamics.
We have recently developed a procedure that offers contamination-free collection of meibomian glands.11) Traditional LC-MS-based bioanalysis has advantages for reproducible and sensitive quantification; however, spatial information is somewhat lost during the extraction process. Whereas, MS imaging has grown in popularity because of its specificity, improved signal sensitivity, high resolution and comprehensive identification. Advanced MS imaging includes matrix-assisted laser desorption ionization (MALDI), desorption electrospray ionization (DESI), and ion mobility. DESI was first described in 2004 and has become a powerful tool for the rapid imaging of analytes in ambient conditions.12) Unlike MALDI, DESI does not require a matrix layer coating. Moreover, DESI can analyze diverse samples with minimal sample preparation, does not require a vacuum, and is suitable for detecting small molecules more effectively with potentially less contamination.13) Hence, DESI imaging could be an excellent complementary method to traditional LC-MS-based bioanalysis and will provide a wealth of spatial information with confident identification. The objective of this study was to precisely determine azithromycin distribution in rabbit eyelids after a single application via LC-MS-based bioanalysis and DESI imaging.
Male Japanese White rabbits were used (1.6–2.1 kg). The rabbits were individually housed in cages in an air-conditioned room and fed a standard laboratory diet (LRC4; Oriental Yeast, Tokyo, Japan). All animals were maintained under a 12 h light–dark cycle with food and water ad libitum. All of the experimental procedures were approved by the Animal Care and Use Committee of Santen Pharmaceutical.
DosingA 50 µL aliquot of 1% azithromycin ophthalmic solution (AzaSite®) was applied to both eyes of albino rabbits. At specified time points, the rabbits were euthanized with an intravenous pentobarbital overdose. The surfaces of the eyes and conjunctiva were rinsed with saline to remove drug particles.
Determination of Azithromycin ConcentrationThe palpebral conjunctiva and the upper eyelids were surgically excised, and the conjunctival epithelium, lid margins and meibomian glands were collected using a recently developed procedure. Concentrations of azithromycin were determined using an LC-MS/MS method developed and validated in our laboratories. Briefly, tissues were homogenized with stainless steel balls (3 mm diameter) in 100 µL purified water for 30 min using a beads homogenizer (Shake Master Auto; Hirata Corporation, Kumamoto, Japan). Then, 400 µL acetonitrile was added. The resulting mixtures were vortexed and centrifuged (11000×g, 10 min, 4°C). A 5 µL aliquot of the supernatant was mixed with 20 µL methanol, 20 µL internal standard (500 ng/mL erythromycin in methanol), and 100 µL purified water–acetonitrile (20 : 80, v/v). The samples were injected into a reversed-phase LC column (XBridge Phenyl, 2.5 µm, 2.1 mm i.d.×30 mm; Waters). The mobile phase consisting of 0.1% formic acid as solvent A and 0.1% formic acid acetonitrile as solvent B was run at a flow rate of 0.25 mL/min using a gradient program (30–43% solvent B for 8 min). LC-MS/MS analysis was performed on a Prominence UFLC system (Shimadzu, Kyoto, Japan) coupled to a triple-quadrupole mass spectrometer (TSQ Quantum Ultra; Thermo Fisher Scientific, Waltham, MA, U.S.A.) with an electrospray ionization (ESI) interface. All of the instrument controls and data processing were performed using LCquan 2.5 and Xcalibur 1.4 (Thermo Fisher Scientific).
DESI Imaging for Azithromycin DistributionTwo hours post-dose, the cornea and upper eyelids were surgically collected from duplicate rabbits, immediately frozen in liquid nitrogen and stored in dry ice or at −80°C until sectioning. The ocular samples were directly mounted on a Leica CM3000 cryomicrotome maintained at −20°C (Leica Microsystems) without embedding processes to avoid matrix interference with MS analysis. The cornea and eyelids were vertically and horizontally sectioned to a thickness of 30 µm and thaw mounted onto standard microscope glass slides. All of the slides were dried by cold air and stored in an air-tight container with silica gel below −20°C until DESI imaging analysis. The DESI analysis were performed using a DESI 2D™ Ion Source coupled with a linear ion trap mass spectrometer (LTQ; Thermo Fisher Scientific). FireFly™ (Prosolia) was used to create imaging files for visualization in BioMAP (Novartis, Basel, Switzerland). Chemical standards for azithromycin were deposited onto the tissue section and used as a sensitivity check. Signal intensity correlated with the relative analyte concentration.
Figure 1 shows the time–concentration profiles of azithromycin in the conjunctival epithelium, lid margin, palpebral conjunctiva and meibomian glands. Azithromycin concentrations were the highest in the conjunctival epithelium, followed by the palpebral conjunctiva, lid margin and meibomian glands in a 5- to 10-fold descending order. Specifically, azithromycin reached a concentration of 1032.8 µg/g of tissue in conjunctival epithelial tissue 30 min after instillation. Palpebral conjunctiva, lid margin and meibomian glands exhibited 226.1, 82.0 and 0.8 µg/g of tissue at 30 min, respectively. All of the tissues maintained a constant azithromycin level between 30 min and 2 h after application.
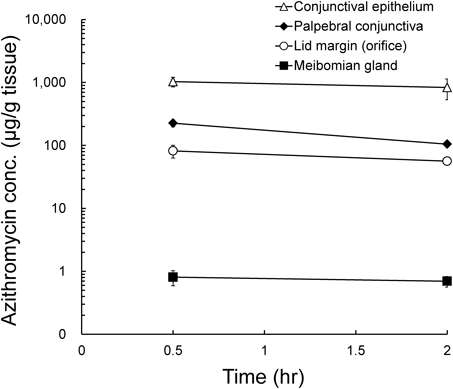
Values represent the means±S.E. (n=4 eyes per group).
The azithromycin distribution in the cornea and eyelids of rabbits was determined using the DESI-MS/MS imaging system and is graphically presented in Fig. 2. The cornea exhibited high signal intensity on the apical epithelium side, but no signal was observed on the basal endothelial side (Fig. 2A). Vertical sections revealed that azithromycin was localized around the eyelash and palpebral conjunctiva but was not present inside the eyelid, including the meibomian glands (Fig. 2B). The horizontal sections showed that a relatively high azithromycin level was associated with the conjunctiva behind the meibomian glands. In contrast, there was no signal within the meibomian glands and lid margins. However, intense signals were detected on the eyelid skin surface (Figs. 2C, D).

Each image represents the relative abundance of azithromycin molecular ion.
Azithromycin is a well-characterized macrolide antibiotic and is presumed to be effective primarily because of its anti-inflammatory and anti-bacterial action.2) For the bacteria commonly involved in bacterial conjunctivitis, intermediate minimum inhibitory concentration MIC90 ranges are reported at less than 4 µg/mL.14) Following the U.S. Food and Drug Administration (FDA)-approved regimen of 1 drop twice daily for 2 d followed by 1 drop daily for 5 d, topical azithromycin reached a maximum concentration of 180 µg/g of tissue in the rabbit eyelid.15) However, our LC-MS-based bioanalysis revealed that azithromycin concentration in the meibomian glands was sub-inhibitory at 0.8 µg/g tissue after a single instillation. This is probably because our protocol enabled precise meibomian gland collection without surface contamination, resulting in much lower concentrations. In contrast to the meibomian glands, the conjunctival epithelium, palpebral conjunctiva and lid margin exhibited higher azithromycin levels than the MIC90; therefore, azithromycin was expected to have an anti-bacterial effect. Azithromycin efficacy might also be attributable to other factors, such as immunomodulation and chronic inflammation. Topical azithromycin application suppressed the expression of pro-inflammatory mediators such as interleukin (IL)-1β, IL-8, and matrix metalloproteinase (MMP)-9 and also increased transforming growth factor (TGF)-β1 expression to normal levels in the lid margin and conjunctiva in human.16) However, impression cytology specimens only contain the several superficial cell layers where drug exposure should be the highest. Indeed, our data showed the highest azithromycin level in the conjunctival epithelium. Previous studies have shown that a minimum of 20 µM azithromycin can prevent the expression of pro-inflammatory mediators and cytokines in vitro.17,18) The minimal concentration of 20 µM was converted to 16 µg/g tissue, assuming that 1 g was equivalent to 1 mL for tissues. Accordingly, azithromycin is expected to exert its anti-inflammatory activity on eyelid surface tissues but not on internal meibomian glands.
Recent studies have demonstrated that azithromycin treatment directly induced lipid accumulation in immortalized human meibomian gland epithelial cells at the concentration of 10 µg/mL.19,20) Indeed, topical azithromycin therapy relieved the signs and symptoms of MGD and restored the biophysical properties of meibomian lipids to normal.5,21) Furthermore, azithromycin topical treatment significantly reduced the number of plugged orifices and ameliorated acinar atrophy in a special diet-induced MGD mouse model.10) These reports support the hypothesis that azithromycin might exert its pharmacological effect directly on the meibomian gland cells to subsequently restore normal lipid properties. However, this hypothesis is based on the assumption that sufficient azithromycin reaches the target cells, whereas the azithromycin concentration in the meibomian glands was only 0.8 µg/g tissue, which was 300- and 1000-fold less than that in the lid margin and conjunctival epithelium, respectively. Overall, topical azithromycin might not achieve sufficient exposure in the meibomian gland to trigger pharmacological activity.
DESI imaging provided more detailed ocular distribution information. In the cornea, intense signals were associated only with the apical epithelial surface, which is consistent with the evidence that azithromycin penetration into the anterior chamber is notably low.22,23) The DESI image visualized the limited azithromycin localization in the conjunctiva, but azithromycin was not detected within tarsal plates.
Having not created an on-tissue calibration curve, DESI imaging provided only relative localization information. Yet, the lower concentration of detection was estimated at approximately 10 µg/g of tissue based on the interpolation of the signal intensity obtained from one eyelid where a known concentration of azithromycin had been applied. This explains why there was no signal observed in the meibomian glands on the DESI image, but was found to contain 0.8 µg/g tissue in the LC-MS-based bioanalysis. Low signal intensity is potentially caused by tissue-specific ion suppression because meibomian glands are a lipid-rich compartment; however, there was no difference in signal intensity between meibomian glands and conjunctiva in the same doped section. These results indicate that signals from the eyelid DESI image are reasonably comparable (data not shown). MS imaging has been used to better understand the molecular changes involved in pathogenic mechanisms in the lens, retina and optic nerve with a variety of resolutions ranging from 10 to 150 µm.24–26) In this study, the spatial resolution was set to 150 µm, which provided sufficient quality images to understand the relative azithromycin abundance in rabbit eyelids; however, MS imaging in smaller tissue, such as mouse eyelids, might require higher resolution to increase granularity, Accoridingly, the limit of detection should be improved by using an exceptional MS system and optimum scan modes.
To our knowledge, this is the first evidence that azithromycin is not homogeneously distributed in the eyelid, suggesting that a tissue barrier might restrict azithromycin penetration across the tarsal plate. It is possible that the concentration in the meibomian glands increases in clinical practice because of repeated dosing. Indeed, azithromycin levels accumulated somewhat following multiple administrations not only in the conjunctiva but also in soft tissues such as muscle and subcutaneous adipose tissue.22,27) Therefore, we cannot exclude the possibility that azithromycin might eventually reach meaningful concentrations in the meibomian glands. It is important, however, to recognize the limited penetration of azithromycin into the meibomian glands. Further studies are required to investigate the effects of repeated dosing on drug distribution in the meibomian glands and the contribution from systemic circulation.
In conclusion, our results have provided important evidence that topically administered azithromycin is predominantly distributed in the conjunctiva and might not be active in the meibomian glands.
Nagayoshi Asano, Fumio Tsuji and Kouichi Kawazu are employees of Santen Pharmaceutical Co., Ltd. Justin Wiseman is the President of Prosolia, Inc.