Abstract
Hand–foot skin reaction is recognized as one of the most common adverse events related to multiple tyrosine kinase inhibitors, but an effective prevention method has not been identified. The chief aim of this study was to find a mechanism-based preventive method for the skin toxicity induced by sorafenib using vitamin C derivatives. The effects of ascorbyl-2-phosphate magnesium (P-VC-Mg) on the molecular and pathological changes induced by sorafenib were investigated in human keratinocyte HaCaT cells. The cell growth inhibition and apoptotic effects of sorafenib were attenuated by P-VC-Mg. Moreover, P-VC-Mg inhibited the decrease of signal transducer and activator of transcription 3 (STAT3) phosphorylation and the expression of apoptosis suppressors treated by sorafenib. HaCaT cells transfected with the STAT3 dominant-negative form (STAT3DN) and STAT3 small interfering RNA (siRNA) combined with P-VC-Mg did not exhibit the attenuation of cell growth inhibition. Interestingly, after exposure to sorafenib in a three dimensional (3D) skin model assay, the basal layer was significantly thickened and the granular and spinous layers became thinner. In contrast, after exposure to sorafenib with P-VC-Mg, the thickness of the basal, granular, and spinous layers was similar to that of the control image. These findings suggest that P-VC-Mg attenuates sorafenib-induced apoptosis and pathological changes in human keratinocyte cells and in the 3D skin model mediated by the maintenance of STAT3 activity.
The treatment for metastasis renal cell carcinoma has led to significant advances by molecular targeted drugs. However, some safety issues have recently emerged. In particular, adverse reactions induced by multiple tyrosine kinase inhibitors (mTKIs) are some of the major causes for the interruption of therapy involving these drugs.1) Dermatological adverse events, also called hand−foot skin reaction, manifest topically in the palmar and plantar regions, and the pathological mechanism underlying these events is unclear. Because there are few treatment options for renal cell carcinoma and hepatocellular carcinoma, the success of therapy is determined by the length of efficient treatment and the management of adverse reactions. Therefore, appropriate management of the side effects will lead to an improvement in not only the QOL, but also the outcome of therapy2,3) Although the dermatological adverse reactions induced by mTKIs are recognized as serious problems in clinical practice, a preventive method based on the pathological mechanism of these drugs has not been established.
Previously, we reported that the inhibition of signal transducer and activator of transcription 3 (STAT3) contributes to the mechanism for keratinocyte toxicity induced by mTKIs,4) and that the development of hand–foot skin reaction induced by mTKIs is associated with STAT3 polymorphisms.5) STAT3 is a well-known transcriptional factor that regulates cell growth, proliferation, inflammation, and apoptosis.6) Interestingly, STAT3 was reported to maintain homeostasis by regulating cell growth and differentiation in the skin.7,8) It was also reported that psoriasis, characterized as epidermal hyperplasia, is associated with STAT3 activity.9) Therefore, STAT3 activity is theorized to be associated with various cutaneous disorders. Basically, the agent rescuing the STAT3 activity topically may be a prophylaxis for mTKI-induced dermatological side effects.
Vitamin C (VC) is essential for synthesizing collagen in human skin and its efficacy for activating skin turnover has been established in the field of cosmetics. Although the drawback of VC is its low permeability into epidermal skin, this problem was overcome by the recent development of VC derivatives.10) Especially, ascorbyl-2-phosphate magnesium (P-VC-Mg) has attracted attention, because it has high permeability to the subcutaneous tissue and high stability on the epidermal skin. Moreover, lipophilic VC was reported to be highly effective in scavenging oxidative stress and suppressing apoptosis compared to hydrophilic VC.11) Interestingly, dehydroascorbic acid was also reported to induce the increase of STAT3 phosphorylation in cardiomyocytes.12) However, there are few reports on how VC affects signal transduction and its anti-oxidative effects in epidermal keratinocyte cells.
Our aim in this study was to evaluate the efficacy of the VC derivative P-VC-Mg on mTKI-induced keratinocyte toxicity. Analysis of the effects of P-VC-Mg on the sorafenib-induced apoptosis and pathological changes were performed using human keratinocyte cell lines and a three dimensional (3D) skin model.
MATERIALS AND METHODS
ChemicalsSorafenib was purchased from LKT Laboratories. P-VC-Mg was purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Hoechst33258 was purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, U.S.A.). 5(6)-Carboxy-2′,7′-dichlorofluorescein diacetate (5(6)-CDCFDA) was purchased from Enzo Life Sciences (Farmingdale, NY, U.S.A.). Glutathione (reduced form) was purchased from Wako Pure Chemical Industries, Ltd.
AntibodiesAntiphosphorylated (antiphospho)-STAT3 at tyrosine 705 (Tyr705), anti-STAT3, anti-survivin, anti-Mcl-1, anti-FLAG, and anti-rabbit horseradish peroxidase (HRP) conjugate immunoglobulin G (IgG) were purchased from Cell Signaling Technology (Boston, MA, U.S.A.). Anti-β-actin was purchased from Sigma-Aldrich Chemical Co.
Cells and Cell CultureHaCaT cells, the human immortalized keratinocyte cell lines, were kindly provided by Professor Norbert Fusenig (German Cancer Research Centre, Heidleberg, German).13) The HaCaT cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Sigma-Aldrich) supplemented with 10% heat-inactivated fetal bovine serum (lot. no. M3496, MP Biomedicals, Solon, OH, U.S.A.) and 100 units/mL of penicillin, 100 µg/mL streptomycin (Life Technologies, Carlsbad, CA, U.S.A.). Cells were seeded into culture flasks, grown in a humidified atmosphere of 5% CO2–95% air at 37°C, and subcultured with 0.05% trypsin–0.02% ethylenediaminetetraacetic acid (EDTA) (Life Technologies).
WST-8 Colorimetric AssayCell viability was evaluated with the WST-8 assay using Cell Counting Kit-8 (Dojindo, Kumamoto, Japan) describing previously.4) WST-8 assay is a convenient technique for cell analysis using a large number of samples. Moreover, WST-8 is a high sensitive reagent for monolayer-cultured cells, because it detects the activity of dehydrogenase on cell membrane surface. Cells (3×103 cells/well) were seeded on 96-well plates and pre-cultured for 24 h. The medium was exchanged for one containing sorafenib at various concentrations, and then cells were incubated for 48 h at 37°C. The culture medium was replaced with a medium containing a WST-8 reagent, and after 3 h, the absorbance in the well was determined at 450 nm with a reference wavelength of 630 nm using a microplate reader (Infinite M200 Pro, Tecan Group Ltd., Switzerland).
Apoptosis AssayApoptosis-mediated cell death of HaCaT cell was examined by a double staining method using fluorescein isothiocyanate (FITC)-labeled Annexin V/propidium iodide (PI) apoptosis detection kit (BD Biosciences, San Jose, CA, U.S.A.) according to the manufacturer’s instructions. Briefly, cells exposed by drugs were washed in phosphate-buffered saline (PBS) twice, incubated with PBS containing FITC-conjugated Annexin V and PI dyes for 30 min at 37°C. After cells were washed in PBS twice, they were incubated with PBS containing 10 µM Hoechst33258 for 30 min at 37°C. The externalization of phosphatidylserine and the permeability to PI were evaluated by IN Cell Analyzer 2000 (GE Healthcare UK Ltd., Buckinghamshire, U.K.). Cells in early stages of apoptosis were positively stained with Annexin V; whereas, cells in late apoptosis were positively stained with both Annexin V and PI.
Western Blot AnalysisProteins in the total cell lysate were extracted from cells treating to each buffer with Cell Lysis Buffer (Cell Signaling Technology) in addition to 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride and 5 µg/mL leupeptin, and separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electrotransferred on a nitrocellulose blotting membrane, Hybond-P membrane (GE Healthcare). Subsequently, the blot was blocked in a wash buffer solution (10 mM Tris, pH 7.5; 150 mM NaCl; and 0.05% Tween 20) containing 5% skim milk. The membrane was soaked overnight in a wash buffer containing specific primary antibodies, followed by incubation with HRP–conjugated secondary antibodies for 1 h. Antibody-bound proteins were visualized by treating the membrane with an enhanced luminol-based chemiluminescent method, which was freshly prepared just before detection. Finally, blot images were acquired using ChemiStage 16-CC (KURABO Industries Ltd., Osaka, Japan). Wherever indicated, the membranes were stripped and reprobed with a different antibody. The intensities of protein bands for the densitometric assay were determined using ImageJ.14)
Plasmid ConstructionpCDNA3-STAT3-Y705F was a gift from Jie Chen (Addgene plasmid # 74434).15) STAT3-DN constructs were transformed into DH-5α competent cells and plasmid DNA was extracted using the QIAGEN® Plasmid Midi Kit (QIAGEN K.K., Tokyo, Japan). Extracted plasmids were purified to a grade appropriate for cell culture using phenol and chloroform and stocked at 1 µg/µL in a freezer until experimental use.
Transient TransfectionTransient transfection of cell lines with expression vectors was performed using the Lipofectamine LTX transfection reagent (Life Technologies) according to the manufacturer’s protocol. In brief, cells were grown in 96-well culture plates or 60 mm culture dish until they reached ca. 80% confluence. The culture medium was replaced with serum-free Opti-MEM (Life Technologies) and cells were transfected with the DNA–lipofectamine complex. HaCaT cells were transiently transfected with 0.1 µg/well of plasmid in 96-well plates.
RNA InterferingRNA interfering was performed by Silencer® Select Pre-Designed & Validated small interfering RNA (siRNA) for human STAT3 (siRNA ID: s743; Applied Biosystems). siRNA used 10 nM at final concentration. Transient transfection of cell lines with siRNA was performed using the HiPerFect Transfection Reagent (QIAGEN) according to the manufacturer’s protocol.
Evaluation of Reactive Oxygen Species (ROS) ProductionCells were cultured in 96-well plates to 70–80% confluence, followed by drug or H2O2 (as the positive control for ROS generation) and glutathione (as the positive control for the inhibition of ROS generation) treatment for 2 h. Cells were incubated with 10 mmol/L carboxy-H2-DCFDA and 1 µg/mL Hoechst33258 for 30 min, and washed twice with D-PBS. Fluorescence imaging and analysis of the ratio of intracellular fluorescence-positive cells were performed with the IN Cell Analyzer 2000.
3D Skin Model AssayEPI-200 purchased from MatTek (Ashland, MA, U.S.A.) was used as the 3D skin model. EPI-200 was preincubated for 24 h before drug exposure. Assay medium under the dermal side was replaced with 20 µM sorafenib for 96 h following preincubation. On the epidermal side, PBS or 1 mM P-VC-Mg was applied at the same time as the drug. Then, cell viability was evaluated with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and the paraffin–embedded 3D skin model was stained with hematoxylin–eosin (H&E) dye. MTT assay is suitable for evaluation of viability of tissue model consist of multilayer-cultured cells, because MTT has high permeability to cell membrane. The thickness of the basal, granular, and spinous layers was measured through image analysis using ImageJ. Layer thickness was calculated based on the mean values of the median thickness from 3 individual visual fields for each experiment.
Statistical AnalysisData are expressed as means±standard deviation (S.D.) The statistical significance of the difference in mean values between the two groups was analyzed using Student t-test, if the variance of the two groups were similar. Otherwise, Student t-test with Welch’s correction was used for the analysis. Comparisons among more than three groups were performed with non-repeated one-way ANOVA, followed by Tukey’s test. p-Values less than 0.05 (two-tailed) were considered statistically significant.
RESULTS
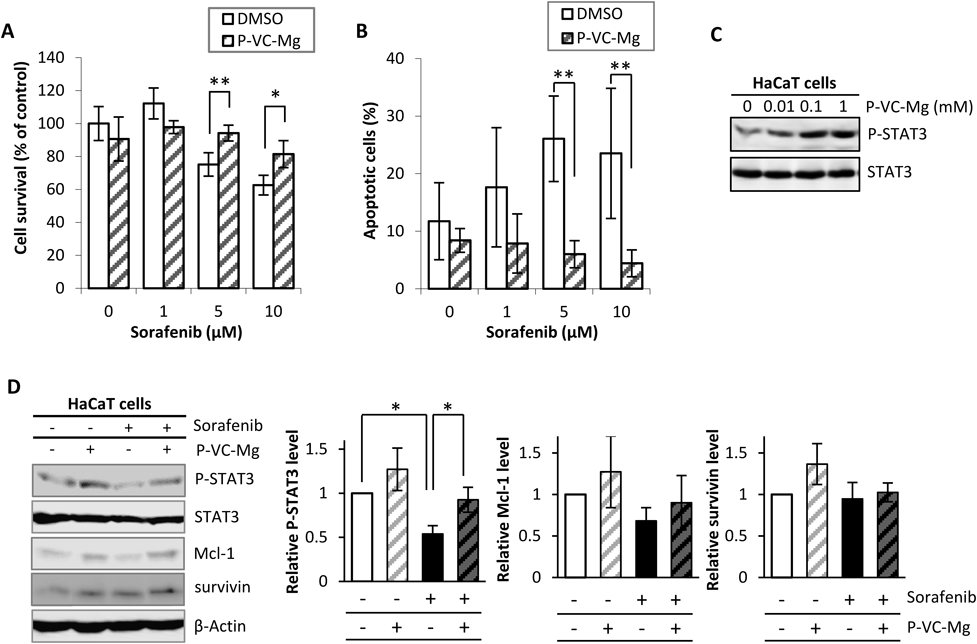
Effects of P-VC-Mg on Cell-Growth Inhibition, Apoptosis, and Signal Transduction Induced by SorafenibFigure 1 shows the sorafenib-induced cell growth inhibition, apoptosis, and signal transduction in HaCaT cells in the absence or presence of P-VC-Mg. The sorafenib-induced cell growth inhibition in HaCaT cells was relieved by co-incubation with P-VC-Mg (Fig. 1A). Additionally, P-VC-Mg reduced the apoptotic effects induced by sorafenib in HaCaT cells (Fig. 1B). P-VC-Mg enhanced the level of phosphorylated STAT3 in a concentration-dependent manner (Fig. 1C) and significantly inhibited the decrease in STAT3 phosphorylation by sorafenib in addition to relieving the decrease in the expression of Mcl-1 (Fig. 1D). P-VC-Mg did not affect survivin expression.
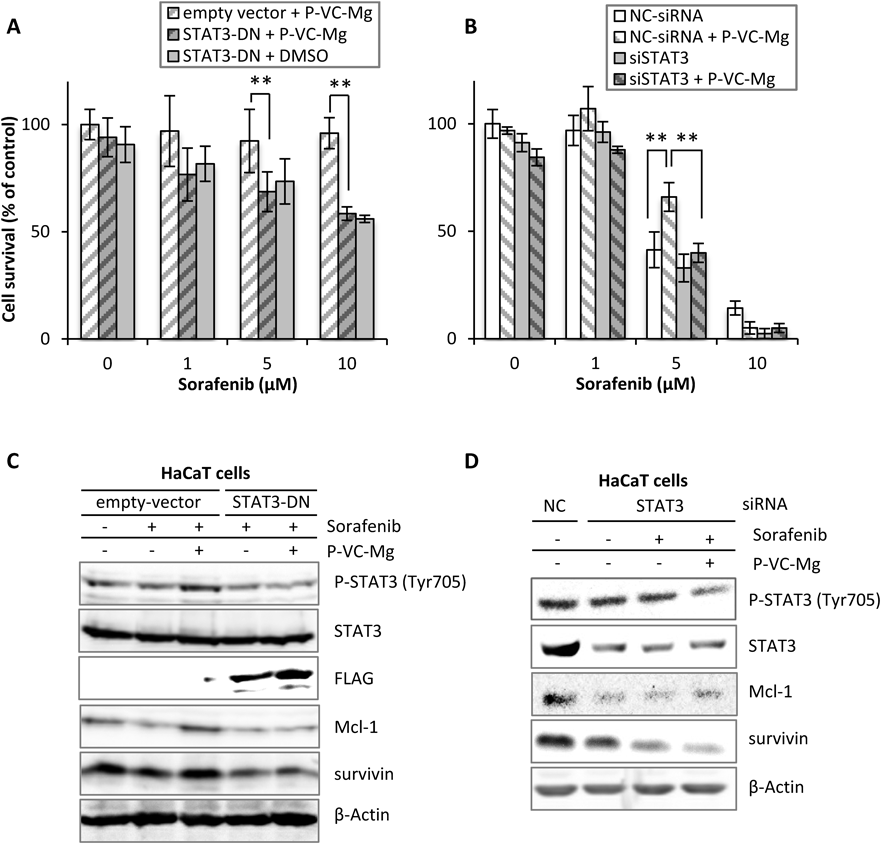
Contribution of STAT3 Activity and Expression to the Recovery Effects of P-VC-Mg for Cell Growth in HaCaT CellsTo confirm that the recovery effects of P-VC-Mg on cell growth are related to STAT3 activation and expression, we experimented using HaCaT cells transfected with STAT3 Y705F, the dominant negative form of STAT3 (STAT3-DN) (Fig. 2A), and STAT3-siRNA (Fig. 2B). Cell growth inhibition by sorafenib was higher with the transfection of STAT3 Y705F than with an empty vector in the presence of P-VC-Mg in HaCaT cells. HaCaT cells transfected with STAT3 Y705F exhibited the same amount of cell growth inhibition by sorafenib in the presence or absence of P-VC-Mg. Additionally, the knockdown of STAT3 enhanced the effects of sorafenib on cell growth inhibition in HaCaT cells, but P-VC-Mg did not attenuate the toxicity of sorafenib to STAT3-knockdown HaCaT cells. Moreover, there was no difference in the expression of Mcl-1 and survivin in cells treated with or without P-VC-Mg in STAT3 Y705F-transfected or STAT3-knockdown HaCaT cells (Figs. 2C, D).
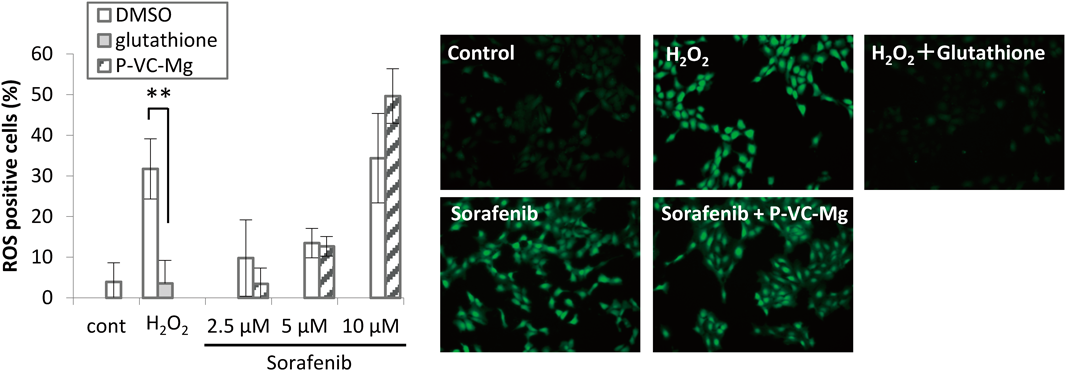
Effects of P-VC-Mg on ROS Production Induced by SorafenibSorafenib-induced significant ROS production in a concentration-dependent manner in HaCaT cells (Fig. 3). However, the ROS production induced by sorafenib was not decreased by P-VC-Mg co-incubation.
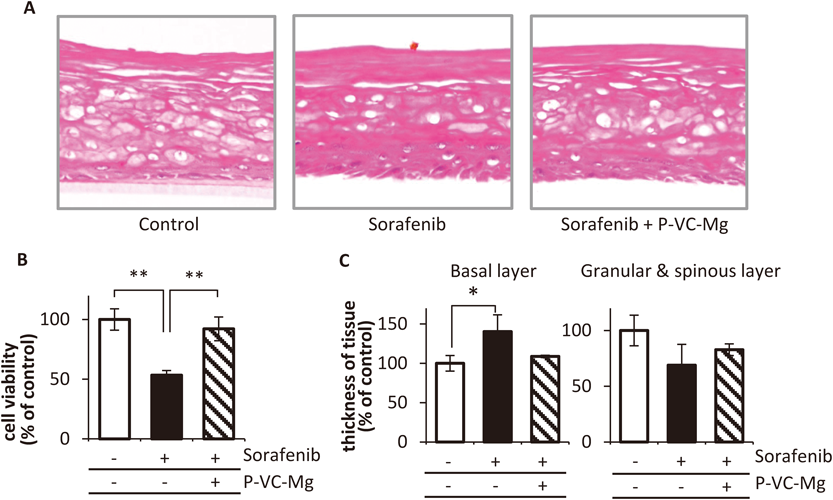
Effects of P-VC-Mg on Pathological Changes by Sorafenib in 3D Skin ModelPathological changes in the 3D skin model following exposure to sorafenib under the dermal side consisted of a significant thickened basal layer, and thinner granular and spinous layers (Figs. 4A, C). However, the images for the exposure to sorafenib under the dermal side and P-VC-Mg treatment on the epidermis side showed that the thickness in the basal, granular, and spinous layers was similar to that in the control histological image. The cell viability in the skin model was recovered by treatment with P-VC-Mg on the epidermis side in spite of the decrease in cell viability induced by exposure to sorafenib on the dermal side (Fig. 4B).
DISCUSSION
Hand–foot skin reaction is generally regarded as the most common problem in multi-kinase targeted therapy. Recently, the efficacy of multi-kinase inhibitors was reported to be correlated with their dermatological toxicity.3,16) However, hand–foot skin reaction can lead to a reduction and discontinuation of treatment and may diminish the effectiveness of therapy. These reports suggest that the management of skin toxicity is essential for the therapeutic success of multiple tyrosine kinase inhibitors.
Our previous study suggested that the keratinocyte toxicity of sorafenib is caused by apoptosis by decreasing the expression of the apoptosis suppressor with mediating decrease of STAT3 activity.4) The results of the present study showed that the sorafenib-induced decrease in the expression of Mcl-1 mediated STAT3 deactivation was attenuated by P-VC-Mg. These results were not observed in HaCaT cells transfected with STAT3-DN or STAT3 siRNA. Therefore, it is suggested that relieving effects of P-VC-Mg on apoptosis by sorafenib contributes to the maintenance of STAT3 activity. Survivin expression was not affected by the level of phosphorylated STAT3, although the reason for this is unclear. Survivin expression is regulated by extracellular signal-regulated kinase and other forms of signal transduction.17) These factors might reduce the expression of survivin induced by P-VC-Mg, but precise experiments are necessary to clarify the relationship between survivin expression and factors that regulate the expression of survivin in the presence of P-VC-Mg.
Dehydroascorbic acid treatment was reported to increase STAT3 phosphorylation in hypoxic cardiomyocytes and ischemic myocardial tissue.12) The authors suggested that STAT3 activation by VC induces enhanced expression of the apoptosis suppressor and reverses ischemic damage. A recent study reported that VC protects STAT3 phosphorylation, possibly mediated by the inhibition of nitrosylation of various receptors.18) Sorafenib is a drug that induces the nitrosylation of cell-death receptors and modulates the production of nitric oxide.19,20) Therefore, the inhibition of nitrosylation by VC may contribute to the protection of STAT3 phosphorylation, but further experiments are necessary to demonstrate our hypothesis.
Sorafenib has also been reported to induce apoptosis through the generation of ROS in various cancer cell lines.21,22) It is well known that VC is a potent antioxidant agent; therefore, we hypothesized that VC scavenges the sorafenib-induced ROS. We evaluated the effects of P-VC-Mg on these mechanisms in keratinocytes. Interestingly, the results of the present study showed that P-VC-Mg does not affect the generation of ROS by sorafenib. However, VC is known to produce ROS when given concomitantly with sulindac,23) and lipid component of ascorbyl palmitate is reported to contribute to the generation of oxidized lipid metabolites that are epidermal cell toxicity.24) Consequently, we expected VC to promote sorafenib-induced apoptosis. However, sorafenib-induced apoptosis in HaCaT cells was suppressed by the VC derivative, suggesting that the repressive effect of the VC derivative on epidermal keratinocyte toxicity induced by sorafenib does not mediate the mechanisms of apoptosis by ROS.
We evaluated the effects of P-VC-Mg on sorafenib-induced changes using pathological images of skin tissue from an in vitro reconstructed human epidermal model in this study, recommended for skin irritation tests in the OECD guidelines.25) The pathological image after treatment with sorafenib under the dermal side in the 3D skin model was similar to images from skin tissue obtained from patients treated with sorafenib who developed severe hand–foot skin reactions.26) The granular and spinous layers appeared shiny and the basal layer was bloated (Figs. 4A, C). These results suggested that this experiment could be a model for hand–foot skin reactions induced by sorafenib. Moreover, treatment with P-VC-Mg on the epidermal side reduced shining in the granular and spinous layers as well as bloating in the basal layer, resulting in an image similar to the non-treatment control model. Furthermore, P-VC-Mg attenuated cell viability reduction in the 3D skin model induced by sorafenib (Fig. 4B). A previous study reported that VC derivatives markedly improved the skin layer structure, including basal cell morphology and abnormal localization of involucrin in the upper suprabasal cells in a reconstructed 3D skin model.27) These results suggest that P-VC-Mg is effective as an animal-testing alternative for keratinocyte toxicity.
STAT3 has a key role in keratinocyte differentiation.28) STAT3 activation in the basal layer has been reported to induce keratinocyte maturation and to increase the amount of keratinocytes in the spinous layer.29) It is possible that STAT3 inhibition induced the converse phenomenon of a thinner spinous layer observed in this study. Therefore, the sorafenib-induced pathogenesis illustrated by the 3D model appears to be a reasonable representation of the hand–foot skin reaction. This study is the first to model dermatological adverse reactions induced by multiple tyrosine kinase inhibitors using a reconstructed human epidermal model in vitro. The model enables the screening of preventive agents for dermatological toxicity induced by various drugs. It would also be helpful for clarifying the mechanism of the development of dermatological toxicity with a focus on specific proteins expressed in each skin layer.
This study had a limited focus on the effects of P-VC-Mg on apoptosis and cell viability. Hand–foot skin reaction is a pathological condition similar to hyperkeratosis and is likely correlated with changes in the expression of cornification proteins. However, this correlation was not evaluated in the present study. More research on this topic is necessary in the future. Moreover, there was a difference between cell viability of HaCaT cells transfected STAT3-DN and STAT3 siRNA in the treatment of sorafenib without P-VC-Mg (Figs. 2A, B), because the cationic lipid transfection reagent for siRNA gave damage and attenuated growth in HaCaT cells (Data are not shown). Searching a novel knockdown reagent with low toxicity to HaCaT cells is necessary.
In conclusion, P-VC-Mg attenuates sorafenib-induced apoptosis in human keratinocytes mediated by the maintenance of STAT3 activity. We established a model for hand–foot skin reaction induced by sorafenib and demonstrated the effects of P-VC-Mg on the skin toxicity induced by sorafenib. P-VC-Mg has potential as a mechanism-based protective agent against hand–foot skin reaction.
Acknowledgments
We thank Mr. Taku Ozawa and Ms. Moeko Kubo (Department of Pharmacy, Kobe University Hospital) for experimental assistance. This work was supported in part by a research grant from the Research Foundation for Pharmaceutical Sciences and the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 24790156 and 26860104.
Conflict of Interest
Dr. Takahiro Ishida is employee of Momotani Juntenkan Ltd., Japan.
REFERENCES
- 1) Lee WJ, Lee JL, Chang SE, Lee MW, Kang YK, Choi JH, Moon KC, Koh JK. Cutaneous adverse effects in patients treated with the multitargeted kinase inhibitors sorafenib and sunitinib. Br. J. Dermatol., 161, 1045–1051 (2009).
- 2) Nardone B, Hensley JR, Kulik L, West DP, Mulcahy M, Rademaker A, Lacouture ME. The effect of hand–foot skin reaction associated with the multikinase inhibitors sorafenib and sunitinib on health-related quality of life. J. Drugs Dermatol., 11, e61–e65 (2012).
- 3) Poprach A, Pavlik T, Melichar B, Puzanov I, Dusek L, Bortlicek Z, Vyzula R, Abrahamova J, Buchler T. Skin toxicity and efficacy of sunitinib and sorafenib in metastatic renal cell carcinoma: a national registry-based study. Ann. Oncol., 23, 3137–3143 (2012).
- 4) Yamamoto K, Mizumoto A, Nishimura K, Uda A, Mukai A, Yamashita K, Kume M, Makimoto H, Bito T, Nishigori C, Nakagawa T, Hirano T, Hirai M. Association of toxicity of sorafenib and sunitinib for human keratinocytes with inhibition of signal transduction and activator of transcription 3 (STAT3). PLOS ONE, 9, e102110 (2014).
- 5) Yamamoto K, Shinomiya K, Ioroi T, Hirata S, Harada K, Suno M, Nishioka T, Kume M, Makimoto H, Nakagawa T, Hirano T, Bito T, Nishigori C, Miyake H, Fujisawa M, Hirai M. Association of single nucleotide polymorphisms in STAT3 with hand–foot skin reactions in patients with metastatic renal cell carcinoma treated with multiple tyrosine kinase inhibitors: A retrospective analysis in Japanese patients. Target. Oncol., 11, 93–99 (2016).
- 6) Darnell JE Jr. STATs and gene regulation. Science, 277, 1630–1635 (1997).
- 7) Quadros MR, Peruzzi F, Kari C, Rodeck U. Complex regulation of signal transducers and activators of transcription 3 activation in normal and malignant keratinocytes. Cancer Res., 64, 3934–3939 (2004).
- 8) Sen N, Che X, Rajamani J, Zerboni L, Sung P, Ptacek J, Arvin AM. Signal transducer and activator of transcription 3 (STAT3) and survivin induction by varicella-zoster virus promote replication and skin pathogenesis. Proc. Natl. Acad. Sci. U.S.A., 109, 600–605 (2012).
- 9) Sano S, Chan KS, Carbajal S, Clifford J, Peavey M, Kiguchi K, Itami S, Nickoloff BJ, DiGiovanni J. Stat3 links activated keratinocytes and immunocytes required for development of psoriasis in a novel transgenic mouse model. Nat. Med., 11, 43–49 (2005).
- 10) Gašperlin M, Gosenca M. Main approaches for delivering antioxidant vitamins through the skin to prevent skin ageing. Expert Opin. Drug Deliv., 8, 905–919 (2011).
- 11) Xiao L, Miwa N. The lipophilic vitamin C derivative, 6-O-palmitoylascorbate protects human keratinocytes and 3D-human skin equivalents against X-ray-induced oxidative stress and apoptosis more markedly than L-ascorbic acid. J. Cell. Biochem., 118, 318–329 (2017).
- 12) Guaiquil VH, Golde DW, Beckles DL, Mascareno EJ, Siddiqui MA. Vitamin C inhibits hypoxia-induced damage and apoptotic signaling pathways in cardiomyocytes and ischemic hearts. Free Radic. Biol. Med., 37, 1419–1429 (2004).
- 13) Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol., 106, 761–771 (1988).
- 14) Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods, 9, 671–675 (2012).
- 15) Kim JH, Yoon MS, Chen J. Signal transducer and activator of transcription 3 (STAT3) mediates amino acid inhibition of insulin signaling through serine 727 phosphorylation. J. Biol. Chem., 284, 35425–35432 (2009).
- 16) Nakano K, Komatsu K, Kubo T, Natsui S, Nukui A, Kurokawa S, Kobayashi M, Morita T. Hand–foot skin reaction is associated with the clinical outcome in patients with metastatic renal cell carcinoma treated with sorafenib. Jpn. J. Clin. Oncol., 43, 1023–1029 (2013).
- 17) Qiu L, Wang Q, Di W, Jiang Q, Schefeller E, Derby S, Wanebo H, Yan B, Wan Y. Transient activation of EGFR/AKT cell survival pathway and expression of survivin contribute to reduced sensitivity of human melanoma cells to betulinic acid. Int. J. Oncol., 27, 823–830 (2005).
- 18) Jang EH, Park CS, Lee SK, Pie JE, Kang JH. Excessive nitric oxide attenuates leptin-mediated signal transducer and activator of transcription 3 activation. Life Sci., 80, 609–617 (2007).
- 19) Caraglia M, Giuberti G, Marra M, Addeo R, Montella L, Murolo M, Sperlongano P, Vincenzi B, Naviglio S, Prete SD, Abbruzzese A, Stiuso P. Oxidative stress and ERK1/2 phosphorylation as predictors of outcome in hepatocellular carcinoma patients treated with sorafenib plus octreotide LAR. Cell Death Dis., 2, e150 (2011).
- 20) Rodríguez-Hernández A, Navarro-Villarán E, González R, Pereira S, Soriano-De Castro LB, Sarrias-Giménez A, Barrera-Pulido L, Álamo-Martinez JM, Serrablo-Requejo A, Blanco-Fernández G, Nogales-Muñoz A, Gila-Bohórquez A, Pacheco D, Torres-Nieto MA, Serrano-Díaz-Canedo J, Suárez-Artacho G, Bernal-Bellido C, Marin-Gomez LM, Barcena JA, Gomez-Bravo MA, Padilla CA, Padillo FJ, Muntané J. Regulation of cell death receptor S-nitrosylation and apoptotic signaling by Sorafenib in hepatoblastoma cells. Redox Biol., 6, 174–182 (2015).
- 21) Park GB, Choi Y, Kim YS, Lee HK, Kim D, Hur DY. ROS-mediated JNK/p38-MAPK activation regulates Bax translocation in Sorafenib-induced apoptosis of EBV-transformed B cells. Int. J. Oncol., 44, 977–985 (2014).
- 22) Wan J, Liu T, Mei L, Li J, Gong K, Yu C, Li W. Synergistic antitumour activity of sorafenib in combination with tetrandrine is mediated by reactive oxygen species (ROS)/Akt signaling. Br. J. Cancer, 109, 342–350 (2013).
- 23) Gong EY, Shin YJ, Hwang IY, Kim JH, Kim SM, Moon JH, Shin JS, Lee DH, Hur DY, Jin DH, Hong SW, Lee WK, Lee WJ. Combined treatment with vitamin C and sulindac synergistically induces p53- and ROS-dependent apoptosis in human colon cancer cells. Toxicol. Lett., 258, 126–133 (2016).
- 24) Meves A, Stock SN, Beyerle A, Pittelkow MR, Peus D. Vitamin C derivative ascorbyl palmitate promotes ultraviolet-B-induced lipid peroxidation and cytotoxicity in keratinocytes. J. Invest. Dermatol., 119, 1103–1108 (2002).
- 25) OECD. “Test No. 439: in vitro Skin Irritation: Reconstructed Human Epidermis Test Method,” OECD Publishing.
- 26) Heidary N, Naik H, Burgin S. Chemotherapeutic agents and the skin: An update. J. Am. Acad. Dermatol., 58, 545–570 (2008).
- 27) Seo A, Kitagawa N, Matsuura T, Sato H, Inai T. Formation of keratinocyte multilayers on filters under airlifted or submerged culture conditions in medium containing calcium, ascorbic acid, and keratinocyte growth factor. Histochem. Cell Biol., 146, 585–597 (2016).
- 28) Hauser PJ, Agrawal D, Hackney J, Pledger WJ. STAT3 activation accompanies keratinocyte differentiation. Cell Growth Differ., 9, 847–855 (1998).
- 29) Albanell J, Rojo F, Averbuch S, Feyereislova A, Mascaro JM, Herbst R, LoRusso P, Rischin D, Sauleda S, Gee J, Nicholson RI, Baselga J. Pharmacodynamic studies of the epidermal growth factor receptor inhibitor ZD1839 in skin from cancer patients: histopathologic and molecular consequences of receptor inhibition. J. Clin. Oncol., 20, 110–124 (2002).