2018 年 41 巻 1 号 p. 20-23
2018 年 41 巻 1 号 p. 20-23
We recently found that 10.5% of sporadic Parkinson’s disease (PD) patients lacked one copy of the midnolin (MIDN) gene. In addition, gene knock-down/out of MIDN caused down-regulation of parkin E3 ubiquitin ligase, indicating MIDN to be a novel PD-risk factor or causative gene. In this study, we performed RNA-sequencing and transcriptome analysis of Midn wild-type and knockout cells. Midn positively or negatively regulated the expression of a wide variety of genes, including causative familial PD genes, such as α-synuclein, parkin, and EIF4G1. However, EIF4G1 protein levels were not altered by the reduction of its mRNA by Midn loss, as seen that parkin protein levels were correlated to the mRNA down-regulation. Taken together, these findings indicate that MIDN regulates the expression of a wide variety of genes, including multiple PD-causative genes and is associated with PD onset.
Parkinson’s disease (PD) is a progressive neurodegenerative disease accompanied by loss of dopaminergic neurons in the substantia nigra region of the midbrain, which projects to the striatum, resulting in an extrapyramidal disorder. Approximately 10% of PD is familial, and many causative genes have been identified.1–3) However, the other 90% of PD occurs sporadically and its causes are poorly understood.
Midnolin (Midn) was discovered using a gene trap approach in embryonic stem cells.4) MIDN is strongly expressed in the midbrain, and is localized to the nucleus and nucleolus. However, its expression is not limited to the midbrain and it is widely expressed in major organs.4) MIDN interacts with glucokinase via an N-terminal ubiquitin-like domain with high homology to parkin ubiquitin E3 ligase, leading to a significant reduction in glucokinase activity and glucose-induced insulin secretion from MIN6 cells.5) Overall, although MIDN was discovered many years ago, its pathophysiological roles remain unclear.
We recently showed that MIDN expression is induced by nerve growth factor (NGF) in PC12 cells, a model rat cell line for catecholaminergic neurons and that NGF-stimulated neurite outgrowth is attenuated in MIDN-deficient cells.6) However, tyrosine hydroxylase protein levels were not significantly altered in the study, indicating MIDN may not be involved in catecholamine biosynthesis in these cells.6) Surprisingly, 10.5% of patients with sporadic PD lacked one copy of the MIDN gene whereas no copy number variation was observed in healthy people, suggesting MIDN to be a novel PD-risk factor or causative gene. Moreover, parkin (Park2), a familial PD-causative gene, was down-regulated by Midn knockout/down in PC12 cells.6) MIDN is assumed to be a transcription modulator; therefore, examination of expression of other PD-related genes in Midn-manipulated cells is warranted. Thus, a comprehensive transcriptome analysis was performed in wild-type and Midn-knockout PC12 cells.
MIDN-positive and -negative PC12 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% heat-inactivated fetal calf serum, 5% horse serum, 50 units/mL penicillin, and 50 µg/mL streptomycin. MIDN-negative PC12 cells were created by clustered regularly interspaced short palindromic repeats (CRISPR/Cas9) gene editing as described previously.6) Total RNA was extracted from cells using TriPure isolation reagent (Roche, Indianapolis, U.S.A.), and used for RNA-seq and quantitative (q)RT-PCR. For RNA-seq, mRNA was purified by removal of ribosomal RNA using a RiboMinus Eukaryote kit for RNA-seq (ThermoFisher Scientific, Rockford, U.S.A.). RNA-seq libraries were then prepared using an Ion Total RNA-Seq Kit Ver2 (ThermoFisher Scientific). Samples were amplified by emulsion PCR and Ion Sphere particles were concentrated. Then, samples were analyzed on a next generation sequencer (Ion Proton, ThermoFisher Scientific). The obtained sequence data were analyzed using CLC Genomics Workbench software 10.0.1 (QIAGEN, Redwood City, U.S.A.). Expression levels were expressed as reads per kilobase of exon per million mapped reads (RPKM). The total read number range in six samples was between 20543713 and 33962329 reads. For qRT-PCR, total RNA was reverse transcribed using an RT-PCR kit (Toyobo, Osaka, Japan), and real-time PCR was performed using a FastStart Essential DNA Green Master for real-time PCR kit (Roche) and a LightCycler Nano thermal cycler (Roche). The PCR primers used are shown in Supplementary Materials 1. PCR products were quantified and are expressed according to fold change. Data are expressed as means±standard error of the mean (S.E.M.), and statistical significance was analyzed using the unpaired Student’s t-test.
We could analyze the expression of 32494 genes by RNA-seq; scatter and volcano plots are shown in Fig. 1. MIDN positively and negatively regulates the expression of a wide variety of genes although MIDN-positive cells showed a tendency to express genes at higher levels. Next, we focused on the expression of PD-causative genes (Table 1). Among the major PD-causative genes, the expression levels of nuclear, ubiquitous, casein kinase and cyclin kinase substrate 1 (NUCKS1/PARK16) and eukaryotic translation initiation factor 4G1 (EIF4G1/PARK18) were significantly reduced by MIDN depletion. α-Synuclein (SNCA/PARK1/4) expression increased by 5.66 fold (p=0.0558) and ATP13A2/PARK9 decreased by −1.55 fold (p=0.0520). The expression of PRKN/PARK2, which encodes parkin, was shown to be significantly reduced by qRT-PCR in a previous study6); however, no significant change was detected by RNA-seq. Note that the change in expression of Midn itself was −1.60 fold by RNA-seq, which was lower than the −4.28 fold determined by qRT-PCR. This is because Midn depletion was performed by the CRISPR/Cas9 system and, even though a frame-shift was artificially created, reads were still mapped onto the down-stream region. To compare and confirm the results, we examined five PD-related genes expressions of which were significantly changed (p<0.05) or close to significance level, and parkin by qRT-PCR (Fig. 2). SNCA/PARK1/4 expression was significantly increased by 7.59 fold. On the other hand, expression of PRKN/PARK2 and PARK18/EIF4G1 was significantly decreased by −2.34 and −1.75 fold, respectively.
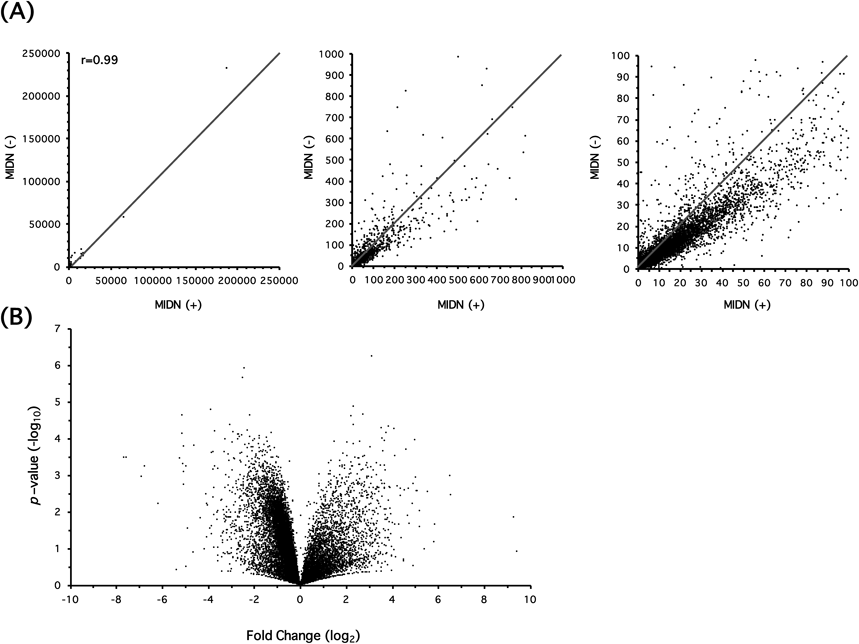
(A) Transcriptome analysis by RNA-seq was performed using MIDN-positive (+) and negative (−) cells, and the scatter plots are expressed in three diverse RPKM scales. (B) Volcano plot analysis. X-Axis indicates fold change in log2 scale. Positive values mean greater expression in MIDN (−)-PC12 cells compared with MIDN (+)-cells and vice versa for negative values. Y-Axis indicates p-value in −log10 scale. There were 875 genes (2.69%) whose expression increased by more than 1.5 fold (p<0.05) in response to Midn depletion whereas the expression of 3680 genes (11.3%) decreased by more than 1.5 fold (p<0.05). When the threshold value was defined as 2.0 fold (p<0.05), expression levels of 768 genes (2.36%) increased while those of 1659 genes (5.11%) decreased.
| Gene name | Fold | p-Value | MIDN (+) | MIDN (−) | |||
|---|---|---|---|---|---|---|---|
| Mean | S.E.M. | Mean | S.E.M. | ||||
| PARK1/4 | SNCA | 5.65621961 | 0.05583822 | 0.01009606 | 0.01009606 | 0.05710551 | 0.01442874 |
| PARK2 | PRKN | −1.62431166 | 0.15129955 | 0.44183014 | 0.07479486 | 0.27201069 | 0.06002149 |
| PARK3 | SPR | 1.32159860 | 0.25064343 | 19.32005917 | 2.59916598 | 25.53336311 | 3.83054683 |
| PARK5 | UCHL1 | −1.19482627 | 0.43765673 | 161.26372586 | 17.11035662 | 134.96834686 | 25.28582408 |
| PARK6 | PINK1 | −1.28220295 | 0.09874657 | 2.80772999 | 0.14074647 | 2.18977034 | 0.25166941 |
| PARK7 | DJ-1 | −1.53290619 | 0.08667070 | 21.16497485 | 2.45647518 | 13.80709070 | 2.13643715 |
| PARK8 | LRRK2 | 1.23490288 | 0.63585138 | 0.03558327 | 0.01592718 | 0.04394188 | 0.00363535 |
| PARK9 | ATP13A2 | −1.54852923 | 0.05197596 | 5.54438362 | 0.49919523 | 3.58041908 | 0.51484509 |
| PARK11 | GIGYF2 | −1.50297522 | 0.07454215 | 4.90070954 | 0.57046250 | 3.26067222 | 0.37740550 |
| PARK13 | HTRA2 | −1.08879795 | 0.61703516 | 12.37062501 | 0.77070722 | 11.36172694 | 1.69700646 |
| PARK14 | PLA2G6 | −1.28928789 | 0.09324874 | 5.04847501 | 0.11306382 | 3.91570809 | 0.50370995 |
| PARK15 | FBXO7 | −1.23634030 | 0.22306793 | 21.99558252 | 1.52409460 | 17.79088048 | 2.48863518 |
| PARK16 | NUCKS1 | −1.90560701 | 0.03585119 | 59.80420988 | 7.82996814 | 31.38328600 | 4.70880804 |
| PARK17 | VPS35 | −1.62833636 | 0.13383485 | 35.48950005 | 6.05658238 | 21.79494417 | 4.07215197 |
| PARK18 | EIF4G1 | −1.82451071 | 0.00944347 | 129.36010404 | 10.82096853 | 70.90125779 | 6.23671172 |
Positive fold values indicate greater expression in MIDN (−)-PC12 cells compared with MIDN (+)-cells and vice versa for negative values (n=3).
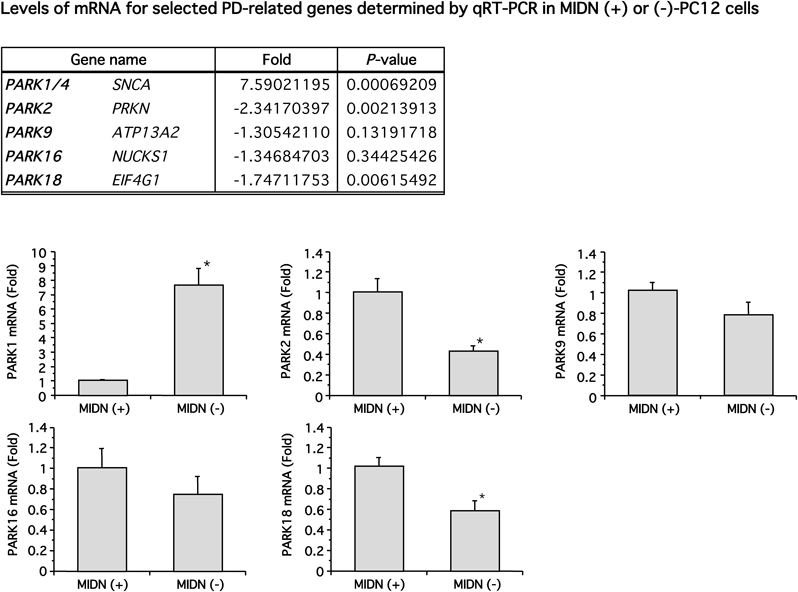
Gene expression of SNCA/PARK1/4, PRKN/PARK2, ATP13A2/PARK9, NUCKS1/PARK16 and EIF4G1/PARK18 was examined by qRT-PCR (n=5–6). Positive fold values indicate greater expression in MIDN (−)-PC12 cells compared with MIDN (+)-cells and vice versa for negative values. The expression of SNCA/PARK1/4, PRKN/PARK2, and EIF4G1/PARK18 was significantly altered by Midn depletion (* p<0.05). A correlation factor for fold changes of these five genes between RNA-seq and qRT-PCR was 0.993.
In the present study, we performed transcriptome analysis to clarify the role of MIDN in gene transcription. RNA-seq showed MIDN altered the expression of various genes positively or negatively (Fig. 1). Because MIDN is primarily localized in the nucleus,4–6) it is assumed to be a transcription factor. However, Midn encodes neither an apparent DNA binding domain nor a transactivation domain, suggesting it functions as a transcription modulator that interacts with unidentified transcription factors to regulate the expression of various genes. The expression of many PD-causative genes was also affected (Table 1). It is interesting that α-synuclein, a major component of Lewy bodies, was up-regulated by loss of Midn, although RPKM values were low, whereas most other genes were down-regulated. After screening by RNA-seq, we confirmed expression levels by qRT-PCR. Three genes were found to be significantly altered by MIDN (i.e., SNCA, PRKN and EIF4G1) (Fig. 2). As reported previously, MIDN copy number loss was observed in patients with sporadic PD and we speculated that reduced Parkin expression resulted in PD onset. However, in addition to Parkin, at least two other genes were candidates as MIDN-regulated genes. α-Synuclein is a major PD-causative gene, and missense mutations and gene multiplication, or excessive phosphorylation of α-synuclein by GRK5 and GRK6 promotes its deposition in Lewy bodies.2,7,8) EIF4G1 is ubiquitous and abundantly expressed in various tissues. It operates as a scaffold protein that interacts with many translation initiation factors.2) Mutations in this gene (R1205H and A502V) cause autosomal-dominant late-onset PD with Lewy bodies.9) However, the frequency of the non-synonymous EIF4G1 variant is less than 1% in the PD population.10) Thus, it is assumed that loss of MIDN affects the expression of multiple PD-causative genes, resulting in the onset of PD. We examined the protein levels of these genes by Western blotting. We have already shown that Midn suppression by CRISPR/Cas9 and small interfering RNA causes down-regulation of Parkin protein expression.6) However, EIF4G1 protein levels were unchanged in PC12 cells (Supplementary Material 2). Because EIF4G1 levels are abundant in many cell types, it is assumed that the changes of the mRNA levels were not reflected to the protein levels in PC12 cells at least. α-Synuclein protein in PC12 cells was not detected in our system. It is known that α-synuclein is a small peptide and is easily detached from membranes, which makes it difficult to detect it by Western blotting. Furthermore, its expression is not ubiquitous but is unevenly distributed in brains and bone marrows (https://www.ncbi.nlm.nih.gov/gene/6622). For these reasons, we assume that α-synuclein protein was not detected in PC12 cells.
In addition to cellular models, it is essential to examine the pathophysiology of Midn knockout mice. Interestingly, parkin knockout mice revealed no accumulation of parkin substrates and normal dopaminergic neurons within the substantia nigra,11–13) indicating the requirement of additional pathogenic events to promote PD. However, because mRNA expression levels of α-synuclein, EIF4G1 and parkin are altered, Midn knockout mice may display a PD-like phenotype.
MIDN is associated with PD, but the relevance to other neurodegenerative diseases such as Alzheimer’s disease and amyotrophic lateral sclerosis has not been examined. In our transcriptome analysis, major causative gene levels for these diseases including amyloid precursor protein, presenilin 2, apolipoprotein E, superoxide dismutase 1, fused in sarcoma/translated in liposarcoma (FUS/TLS) and transactive response DNA binding protein (TARDBP), were not significantly changed, whereas only presenilin 1 was significantly down-regulated (−1.56 fold, p=0.0251). It is necessary to examine the involvement of MIDN in these and other neurodegenerative diseases.
In conclusion, we demonstrated that MIDN altered the expression of a wide variety of genes, including multiple PD-causative genes, suggesting that these alternations can trigger PD onset. It is essential to further examine the role of MIDN in vitro as well as the phenotypes of MIDN-knockout mice.
This work was supported in part by Grants-in-Aid from the Japan Society for the Promotion of Science (No. 15K07963 to Y.O.), the Takeda Science Foundation (Y.O.) and Setsuro Fujii Memorial, the Osaka Foundation for Promotion of Fundamental Medical Research (Y.O.). We thank Naoki Sagehashi (Yamagata University School of Medicine), Haruki Ochi and Yumiko Furukawa (Institute for Promotion of Medical Science Research, Yamagata University School of Medicine) for technical assistance. We thank Jeremy Allen, Ph.D., from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.