2018 Volume 41 Issue 11 Pages 1659-1666
2018 Volume 41 Issue 11 Pages 1659-1666
Lymphangiogenesis, the formation of lymphatic vessels from preexisting ones, promotes cancer growth and metastasis. Finding natural compounds with anti-lymphangiogenic activity will be useful for preventive treatment of lymphatic metastasis. Shikonin, an ingredient of a traditional Japanese and Chinese medicinal herb Lithospermum erythrorhizon, has been widely used in several pharmaceutical and cosmetic preparations, as well as in food colorants. Shikonin has been reported to inhibit lymphangiogenesis in vitro, but the mechanism of inhibition has not been determined. The aim of this study is to investigate the mechanism of anti-lymphangiogenesis of shikonin in primary human lymphatic endothelial cells (HMVEC-dLy). Shikonin, at non-toxic concentrations, significantly inhibited cord formation ability of lymphatic endothelial cells in a dose- and time-dependent manner. Western blotting analysis showed that shikonin decreased nuclear factor-kappaB (NF-κB) activation, as indicated by phosphorylation and nuclear translocation of NF-κB p65, and also reduced both mRNA and protein levels of hypoxia-inducible factor-1 (HIF-1)α. Use of an NF-κB inhibitor (BAY 11-7085) and HIF-1α small interfering RNA (siRNA) transfection revealed that NF-κB activation was upstream of HIF-1α expression, which controls cord formation by HMVEC-dLy. In addition, the reduction of vascular endothelial growth factor C (VEGF-C) and vascular endothelial growth factor receptor-3 (VEGFR-3) mRNA levels were also found in HMVEC-dLy that treated with shikonin. In conclusion, shikonin inhibits lymphangiogenesis in vitro by interfering the NF-κB/HIF-1α pathway and involves in suppression of VEGF-C and VEGFR-3 mRNA expression.
Lymphangiogenesis, the formation of lymphatic vessels from preexisting vessels, plays an important role in tissue-fluid homeostasis, lipid metabolism and immune control.1) Induction of lymphangiogenesis occurs during normal development and in pathological conditions, such as inflammation, wound healing and cancer. The spread of cancer cells to distant sites, called metastasis, by travelling in lymphatic circulation is frequently observed in many types of cancers.2) Compared to blood vessels, the lymphatic vessels have characteristics that are more supportive for cancer metastasis, such as greater permeability due to having thin walls and few tight junctions, absence of basal lamina, and lack of associated pericytes.3) Inhibition of lymphangiogenesis may be a good way to prevent cancer growth and lymphatic metastasis. Since the currently used chemotherapeutic drugs cannot effectively inhibit metastasis due to their significant side effects, discovery of compounds that can inhibit lymphangiogenesis will be important.
Shikonin is a natural product found in the root of Lithospermum erythrorhizon, which is a herb used in traditional Japanese and Chinese medicine. Shikonin has been used as an active ingredient in several pharmaceutical and cosmetic preparations, including as a component of food colorants.4) Shikonin is an interesting natural compound to develop for treatment of cancer diseases, because long-term study of the systemic toxicity of shikonin and derivatives in animal models demonstrated that shikonin should be safe for usage.5) In addition, shikonin possesses multiple pharmacological activities, such as anti-inflammation, anti-cancer, and anti-angiogenesis properties in in vitro and in vivo studies.6,7) Our previous study found that shikonin expressed anti-lymphangiogenesis activity in vitro,8) but the underlying mechanism of shikonin still needs to be investigated.
Nuclear factor-kappaB (NF-κB) is an inducible transcription factor that mediates signal transduction between cytoplasm and nucleus in many cell types. NF-κB activation upregulates genes such vascular endothelial growth factor C (VEGF-C)9) and its receptor, vascular endothelial growth factor receptor-3 (VEGFR-3),10) which are the key promoting factors of lymphangiogenesis and lymph node metastasis.1) NF-κB consists of a family of transcription factors that include five proteins, p50, p52, p65 or RelA, RelB, and c-Rel, that exist as homo- and hetero-dimers. The most common NF-κB heterodimer is composed of p50 and p65.11) NF-κB is normally sequestered in the cytoplasm by forming a complex with inhibitor of kappa B (IκB) protein. When stimulated, IκBα and NF-κB are phosphorylated by IκB kinases (IKKs), and the IκBα/NF-κB complex is subsequently dissociated, the phosphorylated NF-κB translocates to the nucleus where it up-regulates expression of target genes.12) Several mechanisms have been reported for shikonin action in suppressing activation, nuclear translocation, and DNA binding activity of NF-κB.13–16) However, the effect of shikonin on NF-κB function during lymphangiogenesis is still not known.
Hypoxia-inducible factor-1 (HIF-1) transcriptional factor, composed of two subunits, HIF-1alpha (HIF-1α) and HIF-1beta (HIF-1β), can promote or repress the transcription of a broad range of genes that are involved in maintaining biological homeostasis, such as influencing metabolic adaptation, the innate immune response, cell survival, and apoptosis.17,18) The expression of HIF-1α is an important factor during lymphangiogenesis.19) NF-κB has been shown to regulate HIF-1α expression,11,20) and NF-κB activation has been found during lymphangiogenesis.21) Therefore, we hypothesized that shikonin might suppress lymphangiogenesis via interfering with the function of NF-κB and HIF-1α pathway.
In this study, we demonstrated for the first time that NF-κB regulated HIF-1α expression during lymphangiogenesis of human lymphatic endothelial cells in vitro, and shikonin could inhibit the NF-κB/HIF-1α axis and also involved in VEGF-C and VEGFR-3 gene suppression.
Shikonin was purchased from Sigma-Aldrich, cat.no.S7576, Lot SLSL2993V (Saint Louis, MO, U.S.A.), it was dissolved in dimethylsulfoxide (DMSO) to make a stock solution. Matrigel was purchased from BD Biosciences (San Diego, CA, U.S.A.). HIF-1α small interfering RNA (siRNA) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). Purified Mouse Anti-Human HIF-1α was purchased from BD Biosciences (San Jose, CA, U.S.A.). Primary antibodies specific to NF-κB p65 and its phosphorylated form (Ser536) were obtained from Cell Signaling Technology (Beverly, MA, U.S.A.). The polyclonal goat anti-rabbit and anti-mouse immunoglobulin (Ig)/horseradish peroxidase (HRP) secondary antibodies were purchased from Dako (Glostrup, Denmark). NF-κB p65 Immunofluorescence Labeling Kit was purchased from Fivephoton Biochemicals (San Diego, CA, U.S.A.).
Proliferation AssayThe number of surviving cells after treatment was determined by the WST-8 cell proliferation assay kit (DOJINDO, Kumamoto, Japan). Primary human dermal lymphatic microvascular endothelial cells (HMVEC-dLy) cells were cultured in 96-well plates (1.5×104 cells/well), at 37°C. After 24 h, additional medium (100 µL) containing the test sample was added to each well, followed by further incubation for 24 h. After that, 10 µL WST-8 cell proliferation assay kit was added to each well, and incubated continuously at 37°C for 2 h, then the absorbance was measured at 450 nm to determine the number of viable cells.
Lymphatic Endothelial CellsHMVEC-dLy were obtained from Lonza Clonetics (Walkersville, MD, U.S.A.). The cells were cultured in Clonetics EGM-2 MV Bullet Kit (Walkersville, MD, U.S.A.) in a humidified atmosphere (5% CO2, 95% air). Cells were passaged upon reaching 80% confluence. The cells from 5th to 15th passages were used in the study.22)
Cord Formation on MatrigelNinety-six-well plates were coated with Matrigel (10 mg/mL) and allowed to polymerize at 37°C. HMVEC-dLy cells (8×103 cells/well) were seeded on the Matrigel and cultured in medium containing various non-toxic concentrations (0–0.8 µM) of shikonin and incubated at 37°C between 0–8 h. At each the time point of incubation, cells were fixed with a 4% paraformaldehyde solution (Santa Cruz Biotechnology) and stained using hematoxylin solution (Merck KGaA, Darmstadt, Germany). The cord formation network of the cells on Matrigel was photographed. The cord length was measured by using a map meter and expressed as percentage of control.22)
Western Blotting AnalysisCells cultured on Matrigel (40 µg/mL) were washed with phosphate buffer saline (PBS), incubated with RIPA lysis buffer (Santa Cruz Biotechnology), then scrubbed on ice. The collected samples were vortexed, centrifuged at 14000 rpm for 10 min, then supernatant was collected and mixed with loading buffer, boiled for 5 min, and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 7.5–10% polyacrylamide gels. After electrophoretic transferred to a polyvinylidene difluoride (PVDF) membrane, samples were detected by incubation with specific antibodies. The bands were detected by using immunochemiluminescence technique. The β-actin was used as a loading control.
Immunofluorescence MicroscopyThe attached HMVEC-dLy cells after treatment with shikonin on Matrigel (40 µg/mL) were washed with PBS, fixed with 4% paraformaldehyde, and then studied by immunofluorescence method by after staining the cells on Matrigel according to the manufacturer’s instruction. Briefly, the cross-linking reaction was subsequently quenched with a Quenching Buffer (Q-1) (FIVEphoton Biochemicals, U.S.A.). Cells on the slide were permeabilized with a Permeabilization Buffer (P-1) (FIVEphoton Biochemicals) containing a surplus of fish gelatin and bovine serum albumin protein to inhibit non-specific binding of antibody to cells. Then cells were incubated for one hour with primary antibody, p65-specific rabbit IgG (P65Ab) (FIVEphoton Biochemicals). After incubation with primary antibody, the cells were washed and then incubated with secondary antibody, Rhodamine-conjugated anti-rabbit IgG (rRhod) (FIVEphoton Biochemicals) for one hour. Unbound secondary antibody was then washed off, and a sandwich was configured with slide and cover slip and antifade solution; Vectashield mounting medium for 4′-6-diamidino-2-phenylindole (DAPI) staining (Vector, Burlingame, CA, U.S.A.). Flourescence images were captured with a Nikon Eclipse TS100 microscope.
Quantitative RT-PCR (qRT-PCR)Total RNAs were extracted from the HMVEC-dLy cells grown on Matrigel (40 µg/mL) by using TRIzol reagent (Invitrogen, Carlsbad, CA, U.S.A.). The total RNAs at 2 µg were reverse transcribed into cDNA. Subsequently, the PCR reaction was performed by using the StepOnePlus™ (Applied Biosystems, MA, U.S.A.) according to the manufacturer’s instructions. The relative quantification of mRNA expression was calculated as a ratio of the target gene to glyceraldehyde-3-phosphate dehydrogenase (GAPDH), an internal control. The primer sequences were listed as follows: HIF-1α sense, 5′-TTT TTC AAG CAG TAG GAA TTG GA-3′, and antisense, 5′-GTG ATG TAG TAG CTG CAT GAT CG-3′; VEGF-C sense, 5′-TGC CAG CAA CAC TAC CAC AG-3′ and antisense, 5′-GTG ATT ATT CCA CAT GTA ATT GGT G-3′, VEGFR-3 sense, 5′-CTC TGA CCT AGT GGA GAT CCT G-3′ and antisense, 5′-CTT CGG TGA TAT GTA GAG CTG TG-3′, GAPDH sense, 5′-AGC CAC ATC GCT CAG ACA C-3′, and antisense, 5′-GCC CAA TAC GAC CAA ATC C-3′.23)
siRNA TransfectionThe HMVEC-dLy cells were transfected with control siRNA or siRNA against HIF-1α (Santa Cruz Biotechnology) at a final concentration of 6 nM using Lipofectamine RNAiMAX Reagent (Invitrogen). After transfection, the cells were grown for 18 h at 37°C in 5% CO2.19) Subsequently, the transfected cells were harvested and seeded on Matrigel-coated dishes for cord formation assay, Western blotting analysis, and qRT-PCR.
Statistical AnalysisStatistical significance was assessed by one-way ANOVA, with Dunnett’s test and by Student’s t-test. p-Values of less than 0.05 were considered to be significant.
Shikonin significantly decreased cord formation of human lymphatic endothelial cells (HMVEC-dLy), with 16–63% inhibition observed at the non-cytotoxic concentration range (0.1–0.8 µM), in a dose-dependent manner (Figs. 1A, B). Additionally, the inhibition of cord formation by shikonin (0.8 µM) also showed a time-dependent effect over 2–8 h incubation, and a marked inhibition was reached at 6 h incubation (Figs. 1C, D). Thus, shikonin at a concentration of 0.8 µM with 6 h of incubation was selected for further mechanistic study.

The cytotoxic effect of shikonin on HMVEC-dLy was confirmed by using proliferation assay (A). HMVEC-dLy cells were cultured on Matrigel with various concentrations of shikonin for 6 h (B), or with shikonin at a concentration of 0.8 µM for 0–8 h (C). At each time point, photographs were taken, and the relative length of cord-like structures was measured by using a map meter and expressed as percentage compared to Control. Data are expressed as mean±standard deviation (S.D.) of three independent experiments (** p<0.01) compared with Control. (D) Photographs of a representative experiment (Original magnification of 10×).
We further examined the effect of shikonin on activation and nuclear translocation of NF-κB during lymphatic endothelial cord formation. The HMVEC-dLy cells were cultured in medium containing 0.8 µM shikonin on Matrigel-coated dishes for 6 h, then the cells were harvested and subjected to Western blotting analysis and immunofluorescence microscopy. Activation of NF-κB was monitored from NF-κB p65 phosphorylation levels. The results showed that shikonin dramatically suppressed phosphorylation of NF-κB p65 protein compared with control, leading to attenuation of NF-κB activation (Fig. 2A). Amounts of perinuclear accumulation and translocation of NF-κB p65 from cytoplasm into the nucleus were clearly decreased by shikonin treatment (Fig. 2B). These results indicated that shikonin inhibited NF-κB function in human lymphatic endothelial cells during cord formation.

HMVEC-dLy were cultured on Matrigel in the presence or absence of 0.8 µM shikonin for 6 h before analysis. (A) The cord-forming cells were harvested and subjected to Western blotting analysis for detection of total and phosphorylated (active) NF-κB p65 protein, β-Actin was used as a loading Control. (B) Cellular localization of NF-κB p65 protein in the cord-forming cells was detected by immunofluorescence microscopy. Similar results were observed in three independent experiments. (Color figure can be accessed in the online version.)
To confirm the contribution of NF-κB activation in HMVEC-dLy cord formation, cells were treated with NF-κB inhibitor (BAY 11-7085) for 1 h before culturing on Matrigel for 6 h. The results showed that cord formation of the treated cells was significantly decreased, by 71% inhibition compared with control (Fig. 3A), confirming the important role of NF-κB activation in cord formation of human lymphatic endothelial cells.

HMVEC-dLy were treated in the presence or absence of 5 µM NF-κB inhibitor (BAY 11-7085) for 1 h and then cultured on Matrigel for 6 h before analysis. (A) Relative length of cord-like structures was determined from photographs. (B) The cord-forming cells were harvested and subjected to Western blotting analysis for detection of specific proteins. A representative experiment of three independent experiments is shown. (C) HIF-1α mRNA levels was determined by qRT-PCR analysis. Data are expressed as mean±S.D. from three independent experiments (** p<0.01) compared with Control.
Since, it has been reported that NF-κB is a direct modulator of HIF-1α expression,11) we examined the existence of the NF-κB/ HIF-1α pathway in lymphangiogenesis of HMVEC-dLy cells. Western blot analysis revealed that treatment with NF-κB inhibitor caused a decrease in both NF-κB p65 phosphorylation and HIF-1α protein levels (Fig. 3B). qRT-PCR analysis showed that HIF-1α mRNA level in the NF-κB inhibitor-treated cells was significantly reduced, by 0.58-fold reduction compared with control (Fig. 3C).
The results suggested that NF-κB activation was necessary for HMVEC-dLy cord formation. Additionally, NF-κB activation also modulated HIF-1α gene expression during lymphangiogenesis.
Suppression of HIF-1α at Transcriptional Level during Cord Formation by ShikoninSince HIF-1α expression was modulated by NF-κB activation during lymphangiogenesis, we examined the effect of shikonin on HIF-1α protein expression levels during cord formation of HMVEC-dLy on Matrigel. The results showed that amount of HIF-1α protein was dramatically decreased after 6 h of incubation with shikonin (Fig. 4A). We further examined whether the decreased HIF-1α protein level was a result of suppression of HIF-1α gene expression or not. qRT-PCR analysis revealed that HIF-1α mRNA level was significantly decreased in shikonin-treated cells, 0.38-fold decrease compared with control (Fig. 4B). These results suggested that the reduction of HIF-1α protein level in shikonin-treated HMVEC-dLy cells was mediated, at least in part, through inhibition of HIF-1α gene expression.
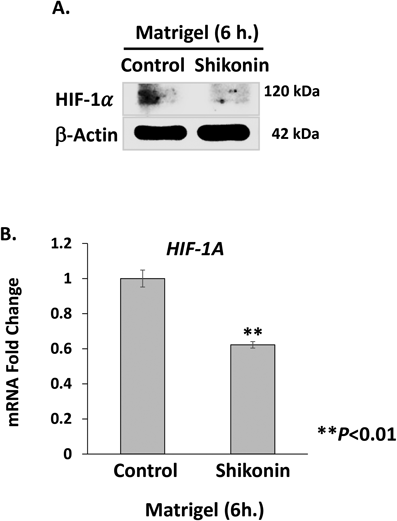
HMVEC-dLy cells were cultured on Matrigel in the presence or absence of 0.8 µM shikonin for 6 h, then harvested and subjected to determination of HIF-1α protein and mRNA levels. (A) HIF-1α protein was detected by Western blotting analysis, β-Actin was used as a loading control. A representative experiment of three independent experiments is shown. (B) HIF-1α mRNA levels were determined by qRT-PCR analysis. Data are expressed as mean±S.D. of three independent experiments (** p<0.01) compared with Control.
The role of HIF-1α in cord formation of HMVEC-dLy cells was confirmed by HIF-1α siRNA silencing. At 18 h after siRNA transfection, the cells were cultured on Matrigel for 6 h, with the result that HIF-1α mRNA expression levels were successfully suppressed by 0.94-fold decrease (Fig. 5A). This correlated with the significant reduction of cord formation by 71% inhibition compared with siControl-transfected cells (Fig. 5B). The results indicated that HIF-1α plays an important role in lymphangiogenesis.
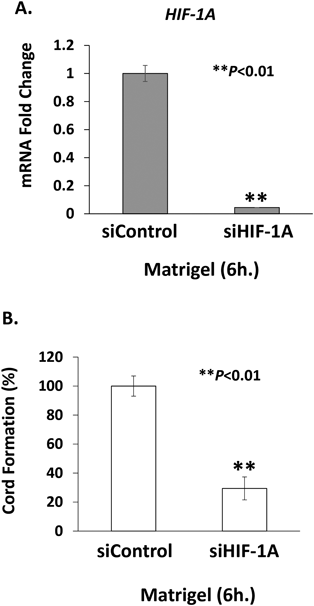
At 18 h after transfection with Control siRNA or HIF-1α siRNA, HMVEC-dLy cells were cultured on Matrigel for 6 h. Then photographs were taken and the cells were harvested and subjected to qRT-PCR analysis. (A) HIF-1α mRNA levels and (B) cord formation after HIF-1α silencing. Data are expressed as mean±S.D. of three independent experiments (** p<0.01) compared with Control.
Taken together, our results demonstrated that NF-κB was an upstream regulator of HIF-1α gene expression in HMVEC-dLy cells during cord formation, and the anti-lymphangiogenic activity of shikonin was mediated by inhibiting NF-κB/HIF-1α pathway.
Effect of Shikonin on VEGF-C and VEGFR-3 Expression during Cord Formation by ShikoninSince, shikonin inhibited NF-κB function in human lymphatic endothelial cells during cord formation (Fig. 2B) and NF-κB activation also upregulates genes such VEGF-C9) and its receptor, VEGFR-3,10) which are the key promoting factors of lymphangiogenesis and lymph node metastasis,1) the suppression of VEGF-C and VEGFR-3 by shikonin may be possible. We further examined the effect of shikonin on VEGF-C and VEGFR-3 mRNA expression levels during cord formation of HMVEC-dLy on Matrigel (Fig. 6). qRT-PCR analysis showed that VEGF-C and VEGFR-3 mRNA levels were significantly decreased in shikonin-treated cells, 0.22-fold (Fig. 6A) and 0.88-fold (Fig. 6B) decrease compared with their controls, respectively. These results suggested that shikonin reduced VEGF-C and VEGFR-3 mRNA levels in HMVEC-dLy during cord formation on Matrigel.

HMVEC-dLy cells were cultured on Matrigel in the presence or absence of 0.8 µM shikonin for 6 h, then harvested and subjected to determination of mRNA levels of (A) VEGF-C and (B) VEGFR-3 by qRT-PCR analysis. Data are expressed as mean±S.D. of three independent experiments (* p<0.05, ** p<0.01) compared with Control.
Although, previous reports found that shikonin, a naphthoquinone derived from the dried roots of Lithospermum erythrorhizon, could inhibit lymphangiogenesis in vitro,8) but its mechanism of action was unknown. The present work is the first report describing the underlying mechanism of anti-lymphangiogenic activity of shikonin.
NF-κB is an inducible transcription factor that regulates expression of many lymphangiogenesis-related genes, including VEGF-C9) and the genes that involved in cell adhesion, cell migration, and cell survival.13,14,16,24) Shikonin has been found to suppress NF-κB function by inhibiting nuclear translocation and DNA binding activity of NF-κB p65 subunit in several cell types.13–16) Shikonin was found to inhibit NF-κB activation and its nuclear translocation during lymphatic endothelial cord formation. Inhibition of NF-κB function by shikonin may affect expression of the lymphangiogenesis-related genes, leading to decrease in cord formation ability of lymphatic endothelial cells.
HIF-1 is an oxygen-regulated transcription factor composed of two subunits, HIF-1α and HIF-1β. HIF-1α protein is constitutively degraded under normal oxygen level condition (normoxia), while it is stabilized under low oxygen condition (hypoxia).17) Heterodimeric HIF-1α/β complex is formed in cytoplasm under hypoxia and subsequently translocated to nucleus where it binds to DNA and promotes expression of lymphangiogenesis inducer genes such as VEGF-A and VEGF-C via direct and indirect pathways.25) HIF-1 has also been reported to play a pivotal role in the cellular response to wound healing, inflammation, and tumor lymphangiogenesis under hypoxic and normoxic conditions.26–28) In our experimental conditions, the HIF-1α protein could be detected in HMVEC-dLy cells during cord formation under normoxia. Growth factors including epithelial growth factors (EGF), fibroblast growth factor-2 (FGF-2), and insulin-like growth factor (IGF) are present in Matrigel,29) and have been shown to induce HIF-1α stabilization in cultured cells under normoxic conditions,18,30) which might contribute to HIF-1α stabilization in lymphatic endothelial cells during cord formation. Shikonin has been reported to be an indirect HIF-1 inhibitor, by blocking HIF-1α expression at transcriptional or translational levels, or promoting degradation of HIF-1α protein.31,32) In our study, shikonin acts as an indirect inhibitor of HIF-1 by suppressing expression of HIF-1α gene, resulting in HIF-1α protein depletion.
NF-κB has been reported to promote HIF-1α gene expression.11,33,34) However, our study is the first to report the relationship between NF-κB and HIF-1α during lymphangiogenesis. Growth factors containing in Matrigel such as insulin-like growth factor (IGF), transforming growth factor-beta (TGF-β), and fibroblast growth factor-2 (FGF-2), have been reported to direct and indirect activate NF-κB in several cell types.29,35–37) After activation, NF-κB translocates to the nucleus and binds to NF-κB-binding site in the promoter region of HIF-1α gene at −197/−188 bp, leading to increased HIF-1α promoter activity and gene transcription.11,38) We demonstrated that an NF-κB inhibitor (BAY11-7085) suppressed NF-κB p65 phosphorylation, resulting in a decrease of HIF-1α mRNA and protein levels, as well as reducing cord formation ability of HMVEC-dLy cells. These findings suggested that NF-κB controlled lymphangiogenesis through, at least in part, modulating HIF-1α expression at transcriptional level.
Since, NF-κB function can also upregulate the other key promoting factors of lymphangiogenesis such VEGF-C9) and its receptor, VEGFR-3.10) Activation of VEGFR-3 by VEGF-C promotes the process of lymphangiogenesis such as lymphatic endothelial cell proliferation, migration, and survival via several signaling pathways.39) We found that shikonin which suppressed NF-κB function and lymphangiogenesis also involved in the inhibition of VEGF-C and VEGFR-3 mRNA expression. However, the details of shikonin on VEGF-C and VEGFR-3 mRNA expression in HMVEC-dLy cells require further proof.
In conclusion, we have demonstrated a mechanism underlying anti-lymphangiogenic activity of shikonin. The compound could inhibit activation and nuclear translocation of NF-κB, leading to decreased HIF-1α gene expression, and resulting in reduction of cord formation ability of lymphatic endothelial cells (Fig. 7). In addition, shikonin also involved in the reduction of VEGF-C and VEGFR-3 gene expression in lymphatic endothelial cells. Our findings suggest the potential benefits of shikonin in suppression of pathological lymphangiogenesis, such as tumor-induced lymphangiogenesis.

When the lymphatic endothelial cells receive signals from its microenvironment, such as growth factors present in Matrigel, which may activate NF-κB signaling leading to up-regulation of HIF-1α gene expression. The Increased HIF-1α levels may enhance expression of the target genes involved in cord formation of lymphatic endothelial cells. Shikonin inhibits activation and nuclear translocation of NF-κB, leading to decreased HIF-1α expression and resulting in reduced cord formation ability of the cells. In addition, shikonin also involves in the down-regulation of VEGF-C and VEGFR-3 gene expression which may be the target genes of NF-κB and resulting in suppressed cord formation ability of the cells as well.
This work was supported by the Faculty of Pharmacy, Srinakharinwirot University, Nakhonnayok, Thailand for research funding [Grant number 248/2560]; [Grant number 580/2560]; and [Grant number 088/2561]. We acknowledge the Laboratory of Biochemistry, Chulabhorn Research Institute, Thailand, the Research Center for Drug Discovery and Development, Faculty of Pharmacy, Srinakharinwirot University, Thailand and the Department of Kampo Diagnostics, University of Toyama, Japan for providing facilities and equipment.
The authors declare no conflict of interest.