Abstract
Taurine has important physiological roles as well as a wide range of pharmacological effects. Studies have suggested that taurine ameliorates diabetes, hypertension, oxidative stress, and inflammatory diseases. However, its mechanisms of action are still unclear. It has been reported that N-acyl taurine activates transient receptor potential vanilloid-1 (TRPV1), which is related to the pathogenesis of many inflammatory diseases. In this study, we hypothesized that taurine has a regulatory effect on TRPV1 activation via N-acyl taurine. To evaluate this hypothesis, we assessed the calcium influx activated by a TRPV1 agonist in human keratinocyte (HaCaT) cells and paraquat-induced oxidative stress in Caenorhabditis elegans. Our results indicate that taurine inhibits TRPV-dependent activity to overcome oxidative stress in cultured cell lines and in C. elegans.
Taurine, or 2-aminoethanesulfonic acid, is an essential amino acid involved in various physiological functions, such as bile acid production, lipid absorption, and neurotransmitter activity.1,2) Further studies have revealed other physiological roles of taurine. It is a key regulator of bile acid-induced inflammation and a crucial substrate of volume-regulated anion channels, which are essential for the regulation of cell volume, growth, division, and migration.3,4) Taurine also pharmacologically ameliorates diabetes, hypertension, oxidative stress, and inflammatory diseases.5–8) It can regulate the gut microbiota to ameliorate inflammatory bowel diseases.9) Furthermore, clinical and mouse studies have revealed that taurine has anti-depressant effects, potentially by promoting neurogenesis, neuronal survival, and growth.10) Although taurine has been examined extensively, its key mechanisms of action are unclear.
N-Acyl amino acids, including N-acyl taurine (NAT), conjugated with several fatty acids are synthesized by several enzymes, such as ACNAT1 and PM20D1.11,12) It has been reported that N-acyl amino acids can increase the proliferation of neuronal progenitor cells and protect neuronal cells against traumatic brain injury.13,14) Saghatelian et al. has revealed that NAT can activate transient receptor potential vanilloid 1 (TRPV1) channel.15) TRPV1 is important in the regulation of immune responses, fat metabolism, and neuronal activity.16–18) In the disease state, the activity of TRPV1 is affected by H2O2, bradykinin, nerve growth factor (NGF) and ATP, and regulates disease responses.19) Therefore, it is important to determine the function of endogenous TRPV1 and its underlying mechanisms.18) Despite evidence for a positive relationship between TRPV1 and NAT, the mechanistic and functional relationship between taurine and TRPV1 activity has not been investigated.
In this study, we found that taurine does not have an agonistic effect on TRPV1 signaling, rather it suppresses TRPV1 signaling induced by NAT as well as another TRPV1 agonist, capsaicin. Notably, taurine extended the longevity of wild-type but not TRPV-mutant Caenorhabditis elegans under oxidative stress, demonstrating that taurine-dependent TRPV inhibition is protective against oxidative stress-induced toxicity in C. elegans. Overall, our results indicate that taurine may be a novel TRPV1 inhibitor and has a potential modulatory effect on the inhibition of TRPV in vivo, which is beneficial for the survival of the organism under oxidative stress condition.
MATERIALS AND METHODS
ReagentsCalcium 6 was obtained from Molecular Devices (San Jose, CA, U.S.A.). Paraquat was obtained from Sigma-Aldrich (St. Louis, MO, U.S.A.). Taurine was obtained from Wako (Osaka, Japan). Antibodies against phospho-acetyl-CoA carboxylase (ACC) (cat #3661), ACC (cat #3662), and phospho-signal transducer and activator of transcription 3 (STAT3) (cat #9145) were obtained from Cell Signaling Technology (Danvers, MA, U.S.A.), and antibody against STAT3 (cat #I1010) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). Horseradish peroxide (HRP)-conjugated secondary antibodies were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA, U.S.A.).
Cell Culture and in Vitro TreatmentHuman keratinocyte cells (HaCaT) were maintained in Dulbecco’s modified Eagle’s medium (DMEM). HaCaT cells were obtained from the American Type Culture Collection (Manassas, VA, U.S.A.). The culture media contained 10% fetal bovine serum from Corning (NY, U.S.A.) and 1% penicillin/streptomycin from Thermo Fisher (MA, U.S.A.).
Western Blotting AnalysisCells were collected and suspended in lysis buffer (25 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), 10 mM Na4P2O7·10H2O), and the protein concentration was quantified using bicinchoninic acid (BCA) assay. Cell protein lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a 10% polyacrylamide gel. Proteins were electroblotted onto a polyvinylidene difluoride (PVDF) membrane (Millipore, Bedford, MA, U.S.A.). Proteins on the PVDF membrane were reacted with specific primary antibodies and HRP-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories). Blots were visualized by chemiluminescence using Super Signal West Pico Chemiluminescence Substrate (Thermo Scientific Inc., Waltham, MA, U.S.A.) and enhanced chemiluminescence (ECL) reagent.
C. elegans Growth Conditions and TreatmentAll C. elegans strains used in the experiments (wild-type strain (N2), TRPV (ocr-1, ocr-2, ocr-3, ocr-4, osm-9) mutant strains (CX4544, CX4544, RB1374, LX950, JY190), and TRPM (gtl2) mutant strain (LH202) were obtained from the Caenorhabditis Genetics Center (University of Minnesota), which is supported by the National Center for Research Resources (MD, U.S.A.). The worms were maintained at 22°C on NGM plates (1.7% agar, 2.5 mg/mL peptone, 25 mM NaCl, 50 mM KH2PO4 (pH 6.0), 5 µg/mL cholesterol, 1 mM CaCl2, 1 mM MgSO4). Escherichia coli OP50 was cultured on NGM plates as a food source. FUdR was included in assays to examine egg-laying defects. For the longevity assay, worms were synchronized by the hypochlorite method and the resulting eggs were seeded on NGM agar plates. For the oxidative stress resistance assay, worms were cultured on paraquat- and taurine-containing NGM plates. Then, worms were evaluated as dead or alive by tapping them with platinum wire every 24 h.
Statistical AnalysisResults are presented as mean±standard error of the mean (S.E.M.). Differences among groups were analyzed by one-way ANOVA with Tukey’s test or Dunnett’s test using StatPlus (AnalystSoft). Life span curves were plotted by using the Kaplan–Meier estimate and analyzed for statistical difference using Log-rank test.
RESULTS
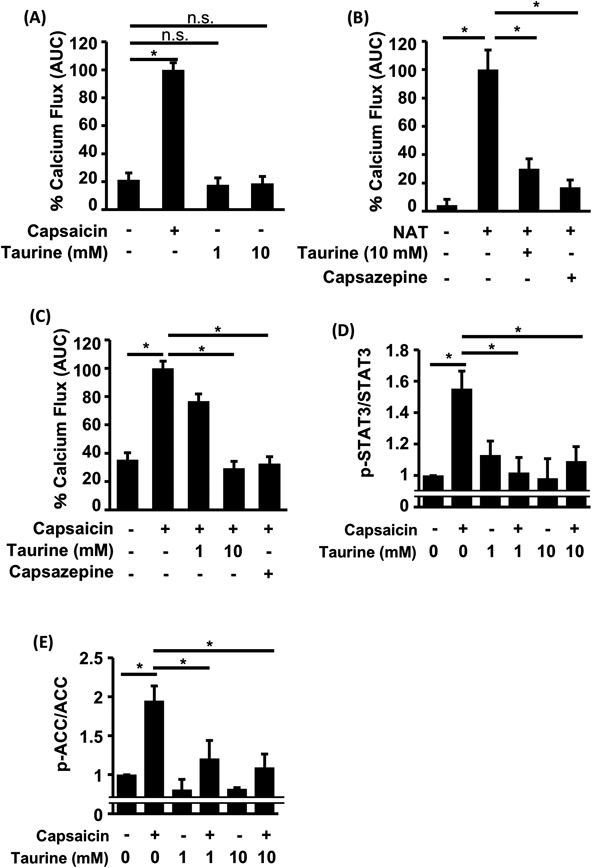
Effect of Taurine on Capsaicin-Induced TRPV1 Ca2+ Influx and SignalingAlthough NAT is a known TRPV1 agonist, little is known about the effect of taurine, a precursor of NAT, on TRPV1 activity. Taurine increases the mitochondrial calcium concentration in myocardial cells,20) but its effect on cytoplasmic calcium homeostasis is unknown. We investigated the effect of taurine using a calcium indicator. In normal conditions, taurine does not affect the basal calcium level (Fig. 1A). The structure of taurine is similar to that of NAT; accordingly, we hypothesized that taurine influences TRPV1 channel activity. To investigate this hypothesis, HaCaT cells were treated with taurine and NAT. Taurine suppressed NAT-induced calcium induction (Fig. 1B) and inhibited TRPV1 activation induced by capsaicin, which is a well-known ligand of TRPV1 (Fig. 1C). The effect of taurine was similar to capsazepine, which is an antagonist of capsaicin and TRPV1. TRPV1 activation was suggested to regulate proinflammatory signaling and metabolic regulating pathways through controlling STAT3 and acetyl-CoA carboxylase (ACC) pathways.21,22) Therefore, we further assessed the physiological function of the taurine-induced inhibition of TRPV1, and found that taurine can inhibit downstream signaling induced by capsaicin (Figs. 1D, E).
Effect of Taurine on the Longevity of C. elegans on Normal Condition and Oxidative Stress ConditionIt is known that taurine has beneficial effects on oxidative stress-induced pathologies in cultured cells and animal models.23–25) To determine the physiological relevance of the TRPV1-inhibitory effect of taurine in vivo, C. elegans was used as an animal model. This organism has a short life span, making it ideal for longevity experiments. Moreover, the effect of taurine on C. elegans has not been examined previously. We first monitored the longevity of taurine-treated wild-type N2 worms in normal conditions. Taurine increased longevity in normal conditions (Fig. 2A). The survival of C. elegans was drastically reduced by paraquat (PQ), an oxidative stress-inducing reagent (Fig. 2B). To assess the effect of taurine on longevity under oxidative stress, C. elegans were treated with taurine in PQ-containing media. Taurine extended the survival of C. elegans in oxidative stress conditions (Fig. 2B). Importantly, based on the data showing that a selective TRPV1 inhibitor capsazepine also dose-dependently reduced PQ-induced oxidative stress damage (Fig. 2C), we confirmed the detrimental role of TRPV1 pathway under oxidative stress condition. Moreover, the shortened life span of C. elegans caused by capsaicin was alleviated by capsazepine, and capsazepine treatment slightly extended C. elegans longevity under normal condition (Fig. 2D). These data supported the idea that TRPV1 activation is detrimental; while TRPV1 inhibition induced by taurine and capsazepine has beneficial effect under both normal and oxidative stress conditions in terms of C. elegans longevity.
The TRPV-Suppressive Activity of Taurine Contributes to the Physiological Effect of Taurine in C. elegansWe next evaluated the mediatory function of TRPV on the physiological effect of taurine in vivo. In several TRPV-mutant C. elegans (ocr-1, ocr-3, ocr-4 and osm-9),26) taurine did not affect longevity in oxidative stress condition (Figs. 3A, C–E). In ocr-2 (Fig. 3B) and in TRPM mutant C. elegans (Fig. 3F), taurine increased the longevity in oxidative stress condition. Taken together, these data indicated that the physiological effect of taurine in C. elegans, i.e., increased longevity, is dependent on TRPV.
DISCUSSION
TRPV1 is a calcium channel present in various cells.18) It is activated by heat, protons, capsaicin and various endocannabinoids. The activation of TRPV1 has been linked to chronic inflammatory conditions and neuropathy. Therefore, TRPV1 inhibitors are potential therapeutic agents for various diseases.18) However, TRPV1 inhibitors are not yet available for routine clinical use.27) The major issue with the development of clinically available TRPV1 inhibitors is severe side effects, such as accidental burns and hyperthermia.27)
Taurine has been used as drug or nutritional supplement. Despite many studies of the effects of taurine in animal models and humans, the mechanism of action is not entirely clear.1) We found that taurine can inhibit the activation of TRPV1, while N-arachidonyl taurine activates TRPV1. The inhibitory effect of taurine on TRPV1 or TRPV can reduce PQ-induced oxidative stress and prolong longevity in stress conditions in vivo. These results suggest that the effects of taurine observed in past studies could be via TRPV1 modulation.
In our study, capsaicin-induced Ca2+ influx was immediately inhibited by taurine treatment. Therefore, extracellular taurine could inhibit TRPV1 activation. Previous reports have shown that extracellular taurine can regulate cellular signaling. For example, taurine promotes angiogenesis via the extracellular signal-regulated kinase (ERK)-Akt signaling pathway when taurine transporter (TauT) is inhibited.28) Intracellular taurine can be conjugated with fatty acids to generate NAT and thereby activate TRPV1.15) The observed effects of long-term taurine supplementation could be explained by TRPV1 activity modulation via the taurine-NAT balance. Taurine can ameliorate diabetes, neuronal dysfunction, and inflammatory bowel disease.8,29,30) TRPV1 activators ameliorate diabetes, and TRPV1 inhibitors or knockout can ameliorate neuronal dysfunction and inflammatory bowel disease.19) Considering the physiological function of TRPV1, the reported effects of taurine may occur through the modulation of TRPV1 function.
In C. elegans, the TRPV subunits OSM-9 and OCR-1, -2, -3, and -4 can form heterotetrameric channels and each has specific function.31) A recent study revealed that nicotinamide could induce cell death in C. elegans by the activation of OSM-9, OCR-4, and heterotetrameric TRPV channels.32) Mutations in OSM-9, OCR-1, -3, and -4 prevent the effects of taurine, but TRPM mutation has no effect. These results suggest that taurine-dependent increase in C. elegans longevity probably depends on OCR-1, 3, 4, and OSM-9 subunits. Interestingly, taurine improved C. elegans longevity better than capsazepine (Figs. 2B, C). These data imply that taurine works as a TRPV inhibitor more effectively in vivo.
We showed that taurine inhibits TRPV1 activation, but the mechanism by which taurine-mediated inhibition of TRPV increases longevity in C. elegans under oxidative stress is still unclear. It is possible that it involves the modulation of mitochondrial activity via TRPV1-dependent Ca2+ influx. Another potential mechanism is via TRPV-cAMP-regulated transcriptional co-activator (CRTC) pathway.33) Previous studies showed that TRPV mutations increased the longevity of C. elegans via the TRPV-CRTC pathway. CRTCs are co-activators of cyclic AMP-responsive element-binding protein (CREB) and are involved in diverse physiological processes including endoplasmic reticulum (ER) stress.34, 35) In ER and heat stress conditions, AMP-activated protein kinase (AMPK) activation reduces CRTC nuclear translocation and prolongs longevity.36) CRTC-AMPK signaling is partly responsible for stress resistance in oxidative stress condition. TRPV inhibition reduces CRTC nuclear translocation and increases the longevity of C. elegans.33) Our results showed that taurine could increase the longevity of C. elegans. The lifespan-prolonging effect of taurine may be via inhibiting the TRPV-CRTC pathway. Further investigations may reveal the mechanism by which the taurine-dependent TRPV reduction increases longevity in oxidative stress conditions.
Acknowledgments
We thank Dr. Yukihiko Sugimoto (Kumamoto University) for providing useful technical advice and assistance. The strains used in this study were provided by the Caenorhabditis Genetics Center (CGC), which is supported by the National Institutes of Health (NIH) National Center for Research Resource. This work was supported by the Program for Leading Graduate Schools HIGO (Health life science: Interdisciplinary and Glocal Oriented; Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan) (to H.K.) and its individual Research Funding project (Fiscal Year 2015–2018 [to M.M.]).
Conflict of Interest
The authors declare no conflict of interest.
REFERENCES
- 1) Ripps H, Shen W. Review: Taurine: A “very essential” amino acid. Mol. Vis., 18, 2673–2686 (2012).
- 2) Ide T, Horii M, Kawashima K, Yamamoto T. Bile acid conjugation and hepatic taurine concentration in rats fed on pectin. Br. J. Nutr., 62, 539–550 (1989).
- 3) Bajor A, Tornblom H, Rudling M, Ung KA, Simren M. Increased colonic bile acid exposure: a relevant factor for symptoms and treatment in IBS. Gut, 64, 84–92 (2015).
- 4) Voss FK, Ullrich F, Munch J, Lazarow K, Lutter D, Mah N, Andrade-Navarro MA, von Kries JP, Stauber T, Jentsch TJ. Identification of LRRC8 heteromers as an essential component of the volume-regulated anion channel VRAC. Science, 344, 634–638 (2014).
- 5) Ito T, Schaffer SW, Azuma J. The potential usefulness of taurine on diabetes mellitus and its complications. Amino Acids, 42, 1529–1539 (2012).
- 6) Anuradha CV, Balakrishnan SD. Taurine attenuates hypertension and improves insulin sensitivity in the fructose-fed rat, an animal model of insulin resistance. Can. J. Physiol. Pharmacol., 77, 749–754 (1999).
- 7) Chen G, Nan C, Tian J, Jean-Charles P, Li Y, Weissbach H, Huang XP. Protective effects of taurine against oxidative stress in the heart of MsrA knockout mice. J. Cell. Biochem., 113, 3559–3566 (2012).
- 8) Marcinkiewicz J, Kontny E. Taurine and inflammatory diseases. Amino Acids, 46, 7–20 (2014).
- 9) Levy M, Thaiss CA, Zeevi D, Dohnalova L, Zilberman-Schapira G, Mahdi JA, David E, Savidor A, Korem T, Herzig Y, Pevsner-Fischer M, Shapiro H, Christ A, Harmelin A, Halpern Z, Latz E, Flavell RA, Amit I, Segal E, Elinav E. Microbiota-Modulated Metabolites Shape the Intestinal Microenvironment by Regulating NLRP6 Inflammasome Signaling. Cell, 163, 1428–1443 (2015).
- 10) Wu GF, Ren S, Tang RY, Xu C, Zhou JQ, Lin SM, Feng Y, Yang QH, Hu JM, Yang JC. Antidepressant effect of taurine in chronic unpredictable mild stressinduced depressive rats. Sci. Rep., 7, 4989 (2017).
- 11) Long JZ, Svensson KJ, Bateman LA, Lin H, Kamenecka T, Lokurkar IA, Lou J, Rao RR, Chang MR, Jedrychowski MP, Paulo JA, Gygi SP, Griffin PR, Nomura DK, Spiegelman BM. The secreted enzyme PM20D1 regulates lipidated amino acid uncouplers of mitochondria. Cell, 166, 424–435 (2016).
- 12) Hunt MC, Siponen MI, Alexson SE. The emerging role of acyl-CoA thioesterases and acyltransferases in regulating peroxisomal lipid metabolism. Biochim. Biophys. Acta, 1822, 1397–1410 (2012).
- 13) Hanuš L, Shohami E, Bab I, Mechoulam R. Hanu: N-Acyl amino acids and their impact on biological processes. Biofactors, 40, 381–388 (2014).
- 14) Waluk DP, Vielfort K, Derakhshan S, Aro H, Hunt MC. N-Acyl taurines trigger insulin secretion by increasing calcium flux in pancreatic beta-cells. Biochem. Biophys. Res. Commun., 430, 54–59 (2013).
- 15) Saghatelian A, McKinney MK, Bandell M, Patapoutian A, Cravatt BF. A FAAH-regulated class of N-acyl taurines that activates TRP ion channels. Biochemistry, 45, 9007–9015 (2006).
- 16) Ninomiya Y, Tanuma SI, Tsukimoto M. Differences in the effects of four TRPV1 channel antagonists on lipopolysaccharide-induced cytokine production and COX-2 expression in murine macrophages. Biochem. Biophys. Res. Commun., 484, 668–674 (2017).
- 17) Baskaran P, Krishnan V, Fettel K, Gao P, Zhu Z, Ren J, Thyagarajan B. TRPV1 activation counters diet-induced obesity through sirtuin-1 activation and PRDM-16 deacetylation in brown adipose tissue. Int. J. Obes., 41, 739–749 (2017).
- 18) Brito R, Sheth S, Mukherjea D, Rybak LP, Ramkumar V. TRPV1: A potential drug target for treating various diseases. Cells, 3, 517–545 (2014).
- 19) Szallasi A, Cortright DN, Blum CA, Eid SR. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat. Rev. Drug Discov., 6, 357–372 (2007).
- 20) Chang L, Zhao J, Xu J, Jiang W, Tang CS, Qi YF. Effects of taurine and homocysteine on calcium homeostasis and hydrogen peroxide and superoxide anions in rat myocardial mitochondria. Clin. Exp. Pharmacol. Physiol., 31, 237–243 (2004).
- 21) Yoshida A, Furube E, Mannari T, Takayama Y, Kittaka H, Tominaga M, Miyata S. TRPV1 is crucial for proinflammatory STAT3 signaling and thermoregulation-associated pathways in the brain during inflammation. Sci. Rep., 6, 26088 (2016).
- 22) Ching LC, Chen CY, Su KH, Hou HH, Shyue SK, Kou YR, Lee TS. Implication of AMP-activated protein kinase in transient receptor potential vanilloid type 1-mediated activation of endothelial nitric oxide synthase. Mol. Med., 18, 805–815 (2012).
- 23) Çetiner M, Sener G, Sehirli AO, Ekşioğlu-Demiralp E, Ercan F, Sirvanci S, Gedik N, Akpulat S, Tecimer T, Yeğen BC. Taurine protects against methotrexate-induced toxicity and inhibits leukocyte death. Toxicol. Appl. Pharmacol., 209, 39–50 (2005).
- 24) Balkan J, Kanbağli O, Hatipoğlu A, Kücük M, Cevikbaş U, Aykaç-Toker G, Uysal M. Improving effect of dietary taurine supplementation on the oxidative stress and lipid levels in the plasma, liver and aorta of rabbits fed on a high-cholesterol diet. Biosci. Biotechnol. Biochem., 66, 1755–1758 (2002).
- 25) Wang GG, Li W, Lu XH, Zhao X, Xu L. Taurine attenuates oxidative stress and alleviates cardiac failure in type I diabetic rats. Croat. Med. J., 54, 171–179 (2013).
- 26) Kahn-Kirby AH, Bargmann CI. TRP channels in C. elegans. Annu. Rev. Physiol., 68, 719–736 (2006).
- 27) Carnevale V, Rohacs T. TRPV1: A target for rational drug design. Pharmaceuticals (Basel), 9, 52 (2016).
- 28) Baek YY, Cho DH, Choe J, Lee H, Jeoung D, Ha KS, Won MH, Kwon YG, Kim YM. Extracellular taurine induces angiogenesis by activating ERK-, Akt-, and FAK-dependent signal pathways. Eur. J. Pharmacol., 674, 188–199 (2012).
- 29) Ito T, Schaffer SW, Azuma J. The potential usefulness of taurine on diabetes mellitus and its complications. Amino Acids, 42, 1529–1539 (2012).
- 30) Menzie J, Pan C, Prentice H, Wu JY. Taurine and central nervous system disorders. Amino Acids, 46, 31–46 (2014).
- 31) Tobin DM, Madsen DM, Kahn-Kirby A, Peckol EL, Moulder G, Barstead R, Maricq AV, Bargmann CI. Combinatorial expression of TRPV channel proteins defines their sensory functions and subcellular localization in C. elegans neurons. Neuron, 35, 307–318 (2002).
- 32) Upadhyay A, Pisupati A, Jegla T, Crook M, Mickolajczyk KJ, Shorey M, Rohan LE, Billings KA, Rolls MM, Hancock WO, Hanna-Rose W. Nicotinamide is an endogenous agonist for a C. elegans TRPV OSM-9 and OCR-4 channel. Nat. Commun., 7, 13135 (2016).
- 33) Riera CE, Huising MO, Follett P, Leblanc M, Halloran J, Van Andel R, de Magalhaes Filho CD, Merkwirth C, Dillin A. TRPV1 pain receptors regulate longevity and metabolism by neuropeptide signaling. Cell, 157, 1023–1036 (2014).
- 34) Wang Y, Vera L, Fischer WH, Montminy M. The CREB coactivator CRTC2 links hepatic ER stress and fasting gluconeogenesis. Nature, 460, 534–537 (2009).
- 35) Crute BE, Seefeld K, Gamble J, Kemp BE, Witters LA. Functional domains of the alpha1 catalytic subunit of the AMP-activated protein kinase. J. Biol. Chem., 273, 35347–35354 (1998).
- 36) Mair W, Morantte I, Rodrigues APC, Manning G, Montminy M, Shaw RJ, Dillin A. Lifespan extension induced by AMPK and calcineurin is mediated by CRTC-1 and CREB. Nature, 470, 404–408 (2011).