2018 Volume 41 Issue 11 Pages 1727-1731
2018 Volume 41 Issue 11 Pages 1727-1731
Duloxetine is a serotonin/noradrenaline reuptake inhibitor that is used as an antidepressant. However, it is known to cause constipation as a side effect. Magnesium compounds, such as magnesium oxide and magnesium hydroxide aqueous solution, are often combined with duloxetine to ameliorate the constipation caused by duloxetine. However, there is concern that these magnesium compounds might alter the effects of duloxetine via physicochemical interactions. In this study, we attempted to clarify the interactions that take place between duloxetine and magnesium oxide using in vivo and in vitro experiments. We evaluated the influence of magnesium oxide on in vitro duloxetine concentrations using HPLC. In addition, we examined the in vivo antidepressant-like effects and serum concentrations of duloxetine in rats. In the in vitro experiment, the duloxetine concentration was significantly decreased by co-treatment with magnesium oxide. In the in vivo experiment, the antidepressant-like effects of duloxetine were not affected by the combined oral administration of magnesium oxide and a duloxetine formulation although the serum duloxetine level was significantly decreased. However, the antidepressant-like effects of a duloxetine reagent were significantly attenuated by the co-administration of magnesium oxide. These results suggest that duloxetine and magnesium oxide directly interact and that such interactions affect the absorption and antidepressant-like effects of duloxetine.
Duloxetine is a serotonin and noradrenalin reuptake inhibitor, and it is clinically used to treat depression, generalized anxiety disorder, diabetic peripheral neuropathic pain, and fibromyalgia. Patients who are undergoing duloxetine treatment often suffer from constipation.1,2) The World Gastroenterology Organization Global Guidelines recommend magnesium hydroxide aqueous solution (milk of magnesia) as one of the first-choice treatments for constipation. In addition, magnesium oxide (MgO) is the laxative that is most commonly used for the prevention and treatment of constipation in Japan.3,4) It is well known that magnesium hydroxide reversibly changes to MgO via dehydration/hydration reactions. Therefore, magnesium compounds are widely used to treat constipation, including constipation caused by duloxetine, around the world.
MgO adsorbs to or chelates with many substances. MgO is reported to adsorb reactive blue 19 dye, iodine, cetyl alcohol, and naphthacene.5,6) In addition, magnesium ions, which are generated when MgO is dissolved, form chelate complexes with dicumarol, such as warfarin, and quinolone antibacterial agents, such as ciprofloxacin.7–9) These physicochemical interactions are considered to interfere with the efficacy of duloxetine by affecting its absorption, but no previous studies have examined the interactions between duloxetine and MgO.
In the present study, we attempted to clarify the interactions that take place between a duloxetine hydrochloride preparation and MgO using in vitro and in vivo experiments. Specifically, we evaluated the in vivo effects of MgO on duloxetine absorption by measuring the serum level of duloxetine. In addition, we assessed the in vivo effects of MgO on the antidepressant-like activity of duloxetine using the forced swimming test, which is a conventional antidepressant screening test. Furthermore, we examined the in vitro effects of MgO on the elution and stability of a duloxetine hydrochloride preparation using HPLC.
In the in vivo experiment, we used duloxetine capsules, containing enteric-coated duloxetine granules (Cymbalta®; Shionogi & Co., Ltd., Osaka, Japan), a duloxetine reagent (Sigma-Aldrich Japan, Tokyo, Japan), and MgO (Sigma-Aldrich Japan).
To assess the in vitro interactions that take place between duloxetine solutions and MgO, we used duloxetine hydrochloride (Wako Pure Chemical Industries, Ltd., Osaka, Japan) and MgO (Sigma-Aldrich Japan). Each chemical was dissolved or suspended in distilled water.
Effects of Magnesium Oxide on the in Vitro Duloxetine ConcentrationPreparation of the in Vitro Duloxetine SamplesDuloxetine solution (2 mg/mL) was mixed with the same volume of distilled water or 33 mg/mL of an MgO suspension. The theoretical final concentrations of duloxetine and MgO were 1 and 16.5 mg/mL, respectively. The duloxetine concentration of the centrifuged supernatant was measured at 0–120 min after the mixing procedure using HPLC.
Next, we measured the duloxetine concentration after adjusting the pH of the duloxetine and MgO mixture because orally administered duloxetine and MgO are exposed to gastric acid. We added hydrochloric acid to the duloxetine (1 mg/mL) and MgO (16.5 mg/mL) mixture to produce three different pH conditions. In group A, an equal volume of hydrochloric acid (pH 1.2) was added to the mixture (final pH: 9.9). In group B, concentrated hydrochloric acid was added so that the pH of the mixture fell to 1.2. In the control group, an equal volume of distilled water was added (final pH: 10.5). In each group, the duloxetine concentration of the centrifuged supernatant was measured 10 min after the mixing procedure using HPLC.
Chromatographic SystemThe samples were analyzed using the Prominence HPLC system (Shimadzu, Kyoto, Japan) with ultraviolet spectrophotometric detection (SPD-20 A, Shimadzu). Chromatographic separation was achieved using the gradient elution method at 40°C on a LiChrospher 125-4.0 Superspher® 100 RP-18e column (125×4 mm, particle size: 4 µm; Kanto Chemical, Tokyo, Japan). The mobile phase used for the gradient elution had the following composition: A: acetonitrile, B: 0.05 M phosphate buffer (pH 3.8). The proportion of component A was kept at 35% between 0 and 3 min and was subsequently increased to 65% from 3 to 8 min. We injected 100-µL samples into the HPLC system. The flow rate was 1.3 mL/min, and the wavelength of the ultraviolet detector was set at 230 nm.
Effects of Magnesium Oxide on the in Vivo Antidepressant-Like Activity and Serum Concentration of Duloxetine in RatsAnimalsMale Wistar rats (Charles River, Japan), weighing 180–200 g, were used for the forced swimming test. Two animals were housed per plastic cage (26×36×25 cm). The room temperature was maintained at 22±2°C, and the rats were kept under a 12-h light/dark cycle (lights on at 8:00). Food and water were provided ad libitum, except during the experiment. The experimental protocol was conducted according to the guidelines of the ethics review committee for animal experimentation of Okayama University Graduate School of Medicine, Dentistry, and Pharmaceutical Sciences.
Drug AdministrationFirst, we conducted a forced swimming test to evaluate the effects of MgO on the antidepressant-like activity of duloxetine using a duloxetine formulation. Duloxetine capsules were de-capsuled, finely pulverized, and then dissolved in distilled water before being used. MgO was suspended in distilled water before being used. The drugs were administered without being mixed; i.e., the animals were administered duloxetine first and then were given MgO just after the duloxetine.
Next, we used the duloxetine reagent to evaluate whether duloxetine directly interacts with MgO. Duloxetine and MgO were suspended separately in 1% carboxymethyl cellulose solution. The duloxetine reagent and MgO were mixed before being administered.
According to the method described in a previous report about the forced swimming test, both drugs were administered orally 3 times (at 24, 3, and 1 h before the experiment) at a dose of 10 mL/kg body weight.10)
Forced Swimming TestForced swimming experiments were carried out according to a previously reported method.11) Black plastic cylinders (height: 37 cm, diameter: 15.5 cm), containing 20 cm of water at 25°C, were used. Two swimming sessions were conducted: an initial 13 min of adaptation swimming and 6 min of test swimming 24 h later. Duloxetine (20, 40, or 80 mg/kg) and MgO (1320 mg/kg) were administered orally at 24, 3, and 1 h before the test swimming. The total period of immobility during the 6-min testing period was recorded using the TARGET series/7 M analysis program (Neuroscience, Tokyo, Japan). Six rats per group were used for the experiment. We used the duloxetine formulation in order to mimic the clinical situation, whereas we used the duloxetine reagent to evaluate whether direct in vivo interactions take place between duloxetine and MgO. Immediately after the 6-min swimming test using the duloxetine formulation, the rats were decapitated, and blood samples were collected and used to measure the serum duloxetine level.
Effects of Magnesium Oxide on the Serum Duloxetine Concentration in RatsThe collected blood samples were centrifuged, and the duloxetine concentrations of the resultant serum samples were measured. Proteins were removed from the serum samples by treating them with the same volume of acetonitrile. After being centrifuged at 13000×g for 15 min, the supernatant was injected into an HPLC column. The HPLC apparatus and analysis parameters were the same as described above in the in vitro section.
Data AnalysisFor the in vivo experiments, values are shown as group means together with standard error of the mean (S.E.M.) values. We used one-way ANOVA followed by Tukey’s test for comparisons among ≥3 groups and the Student’s t-test for comparisons of 2 groups. For the in vitro experiment, values are shown as group means together with standard deviation (S.D.) values. Duloxetine concentrations were calculated from a calibration curve. The time×group interaction was analyzed using two-way ANOVA. One-way ANOVA was used to analyze the influence of acidification on the interactions between duloxetine and MgO. Post-hoc tests were used in combination with Tukey’s test in both analyses.
The effects of MgO on the in vitro duloxetine concentration are shown in Fig. 1. The time courses of the changes in the duloxetine concentrations of the mixtures of duloxetine and water/the MgO suspension are shown in Fig. 1a. There was no time×group interaction. The duloxetine concentration of the duloxetine and MgO suspension mixture was significantly reduced for the whole of the 2-h experimental period [time: F(6, 42)=0.67, p=0.67; group: F(1, 42)=738.28, p<0.01; time×group interaction: F(6, 42)=0.63, p=0.71].
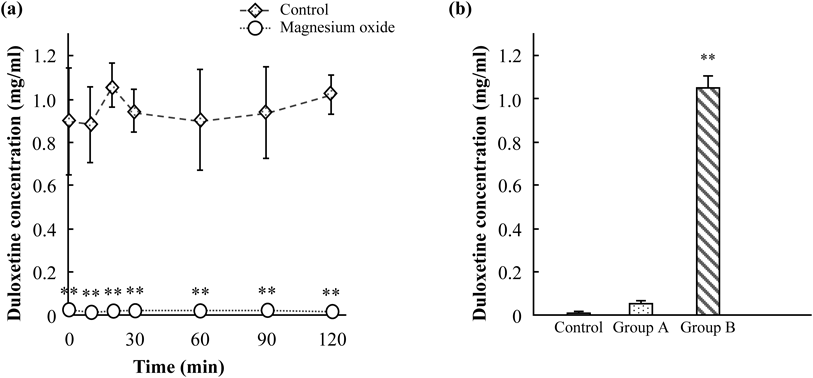
(a) Time course of the changes in the duloxetine concentration with or without MgO. The duloxetine concentration was greatly reduced by MgO. Duloxetine was dissolved in water or a 16.5-mg/mL MgO suspension. The final pH levels of these solutions were 6.5 and 10.5, respectively. The duloxetine concentration at each time point was determined by HPLC. Each value represents the mean±S.D. of 4 samples. Data were analyzed by two-way ANOVA followed by Tukey’s test. ** p<0.01, significantly different from the water group. (b) Acidification attenuated the interaction between duloxetine and MgO. Hydrochloric acid was added to a mixture of duloxetine and MgO, and the final pH levels of these solutions were 10.5 (control), 9.9 (group A), and 1.2 (group B). The duloxetine concentration of each group was determined by HPLC. Each value represents the mean±S.D. of 4 samples. Data were analyzed by one-way ANOVA followed by Tukey’s test. ** p<0.01, significantly different from the control group. Cont.: control group.
In addition, we evaluated the effects of acidification on the interaction between duloxetine and MgO (Fig. 1b). The concentration of duloxetine increased as the pH decreased, and it was significantly increased at pH 1.2 [F(2, 9)=1440.58, p<0.01].
Effects of Magnesium Oxide on the Antidepressant-Like Effects of Pulverized Duloxetine CapsulesThe effects of duloxetine on the immobility time in the forced swimming test are shown in Fig. 2. Duloxetine dose-dependently decreased the immobility time, and the immobility time was significantly decreased at a duloxetine dose of 80 mg/kg [Fig. 2a, F(3, 20)=3.77, p<0.05]. The co-administration of MgO (1320 mg/kg) slightly extended the immobility time, but the difference was not significant [Fig. 2b, F(2, 12)=9.03].

(a) Duloxetine dose-dependently decreased the immobility time in the forced swimming test. (b) MgO did not significantly affect the duloxetine-induced reduction in the immobility time. Each value represents the mean±S.E.M. of 4–6 rats. Data were analyzed by one-way ANOVA followed by Tukey’s test. ** p<0.01 and * p<0.05, significantly different from the control; MgO: 1320 mg/kg magnesium oxide.
The effects of MgO on the duloxetine-induced reduction in the immobility time in the forced swimming test are shown in Fig. 3. The duloxetine reagent significantly decreased the immobility time, as was found for the duloxetine formulation [Fig. 3, F(3, 17)=5.32, p<0.01]. In addition, the co-administration of MgO (1320 mg/kg) significantly reversed the duloxetine-induced reduction in the immobility time (p<0.05). MgO itself did not significantly affect the immobility time.

MgO significantly attenuated the duloxetine-induced reduction in the immobility time. Each value represents the mean±S.E.M. of 5–6 rats. Data were analyzed by one-way ANOVA followed by Tukey’s test. ** p<0.01, significantly different from the control group; † p<0.05, significantly different from the duloxetine alone group; MgO: 1320 mg/kg magnesium oxide.
The serum duloxetine concentration measurements obtained immediately after the test swimming in the forced swimming test and the effects of MgO on the serum duloxetine concentration are shown in Fig. 4. Figure 4a shows that the serum duloxetine level increased dose-dependently, and the mean serum duloxetine concentrations at doses of 40 and 80 mg/kg were 494±55.15 and 722.71±90.57 ng/mL, respectively. The co-administration of MgO (1320 mg/kg) significantly decreased the serum duloxetine concentration (t-value=6.55, p<0.01), and the mean duloxetine concentration in the co-administration group was 585.69±24.59 ng/mL (Fig. 4b).

(a) The serum duloxetine concentration increased dose-dependently. (b): MgO significantly reduced the serum duloxetine concentration. The duloxetine concentrations were determined by HPLC. Each value represents the mean±S.E.M. of 6 rats. Data were analyzed using the Student’s t-test. ** p<0.01, significantly different from the duloxetine alone group.
In the present study, we investigated the interactions that take place between duloxetine and MgO using in vitro and in vivo experiments. In the in vitro experiment, duloxetine almost disappeared after the addition of MgO to duloxetine solution. In the in vivo experiment, although the antidepressant-like effects of pulverized duloxetine capsules were not significantly affected, the serum duloxetine concentration was decreased by the co-administration of MgO. It is widely known that little MgO is absorbed after the oral administration.12) Therefore, the reduction in the serum duloxetine concentration observed in this study was considered to have been due to an intestinal interaction during the absorption process.
In the in vitro experiment, duloxetine almost completely disappeared for the whole of the 2-h experimental period. Therefore, the reduction in the serum duloxetine concentration seen during the forced swimming test would have been caused by decreased absorption due to direct reactions between duloxetine and MgO, rather than by in vivo pharmacokinetic interactions. In the clinical setting, the dosage of MgO used to treat constipation is generally about 1000–2000 mg/d,13,14) and the standard dose of duloxetine is about 40–60 mg/d. Therefore, the dose ratio of MgO to duloxetine is about 16.7–50. Thus, the concentration ratio of MgO to duloxetine employed in the present in vitro experiment is considered to be reasonable.
In the in vivo experiment, duloxetine dose-dependently decreased the immobility time, and it was significantly decreased at a duloxetine dose of 80 mg/kg. It was previously reported that duloxetine exhibited antidepressant-like effects at a dose of 40 mg/kg, whereas we did not observe significant antidepressant-like effects at a duloxetine dose of 40 mg/kg.15,16) These discrepancies might have been due to differences in the experimental conditions and/or analytical parameters; therefore, it remains possible that duloxetine has antidepressant-like effects at a dose of 40 mg/kg.
Next, we studied the influence of 1320 mg/kg MgO on the antidepressant-like effects of 80 mg/kg duloxetine. The antidepressant-like effects of duloxetine were decreased by the co-administration of MgO when we used a duloxetine reagent, but no significant influence of MgO was observed when a duloxetine formulation was used. The MgO dosage for oral administration was estimated from the MgO/duloxetine concentration ratio in the in vitro experiment. Therefore, it is assumed that MgO and duloxetine interacted sufficiently at these dosages, as was the case in the in vitro conditions. It is considered that additives, such as hypromellose, hydroxypropyl methylcellulose acetate succinate, sodium lauryl sulfate, talc, and titanium dioxide, might have attenuated the interactions between duloxetine and MgO. In addition, differences in the experimental methods, such as in the administration methods, might have resulted in the discrepancies between the results obtained with the duloxetine formulation and duloxetine reagent. It is worth noting that the results obtained in this study represent the first evidence that the efficacy of duloxetine might be reduced by the frequent co-administration of a widely used laxative.
In the in vivo experiment, the serum duloxetine level was significantly reduced by MgO, as shown in Fig. 4b. It was reported that the blood duloxetine concentration and the brain target occupancy of duloxetine are almost correlated in humans.17) Therefore, it is assumed that the co-administration of MgO and duloxetine decreased the serum concentration and brain target occupancy of duloxetine, resulting in a reduction in its antidepressant-like effects. It is worth noting that the serum duloxetine concentration was higher when 80 mg/kg duloxetine were administered with 1320 mg/kg MgO than when 40 mg/kg duloxetine were administered alone in the forced swimming experiments using pulverized capsules. Therefore, the serum duloxetine level observed after the co-administration of MgO and duloxetine was considered to be sufficient to exert antidepressant-like effects in the present study. To clarify whether the reduction in the serum duloxetine level was mediated by pharmacokinetic interactions or direct interactions, we evaluated the influence of MgO on the in vitro duloxetine concentration.
In the in vivo experiment, the serum duloxetine level did not decrease markedly after the co-administration of MgO, which disagrees with the results of the in vitro experiment. Duloxetine was able to react with MgO in all of the experiments involving capsules or reagents because the enteric duloxetine granules were finely pulverized. It is considered that drug interactions that are observed in vitro can be affected by various in vivo factors, such as gastric acid; intestinal peristalsis; and distribution coefficients, e.g., for MgO, the contents of the digestive tract, and the gastrointestinal mucosa. In fact, Kumar et al. reported that the adsorption rate of doxorubicin by MgO and zinc oxide nanoparticles was decreased by acidification in an in vitro experiment.18) We also found that the interactions between duloxetine and MgO were attenuated by acidification using hydrochloric acid (Fig. 1b). Therefore, the interactions between duloxetine and MgO might be weakened in the stomach.
However, a reduction in the serum duloxetine level could have significant effects in the clinical setting, even if the interaction between duloxetine and MgO was attenuated. In a previous study, interindividual differences in the serum duloxetine concentration were seen, even when the same dose of the drug was administered, and the responders exhibited higher serum duloxetine concentrations than the non-responders.19) Thus, reductions in the serum duloxetine concentration might lead to the loss of antidepressant effects. Moreover, in some clinical situations, such reactions might occur before the administration of the drug, e.g., when the drugs are administered in a pulverized form or administered using simple suspension method. Thus, MgO might attenuate the antidepressant effects of duloxetine in these clinical situations.
In conclusion, we demonstrated that the antidepressant duloxetine and the laxative MgO interact in vitro. As this interaction between duloxetine and MgO reduced duloxetine absorption, the antidepressant-like effects of duloxetine were reversed by its co-administration with MgO in a rat experimental model. MgO might interfere with the antidepressant-like effects of duloxetine in complex clinical situations, and so care must be taken when co-administering these drugs.
This study was supported in part by JSPS KAKENHI Grant No. 25928004.
The authors declare no conflict of interest.