Abstract
Interleukin (IL)-19 is a member of the IL-10 family of interleukins and is an immuno-modulatory cytokine produced by the main macrophages. The gastrointestinal tissues of IL-19 knockout mice show exacerbated experimental colitis mediated by the innate immune system and T cells. There is an increasing focus on the interaction and relationship of IL-19 with the function of T cells. Contact hypersensitivity (CHS) is T cell-mediated cutaneous inflammation. Therefore, we asked whether IL-19 causes CHS. We investigated the immunological role of IL-19 in CHS induced by 1-fluoro-2,4-dinitrofluorobenzene as a hapten. IL-19 was highly expressed in skin exposed to the hapten, and ear swelling was increased in IL-19 knockout mice. The exacerbation of the CHS response in IL-19 knockout mice correlated with increased levels of IL-17 and IL-6, but no alterations were noted in the production of interferon (IFN)γ and IL-4 in the T cells of the lymph nodes. In addition to the effect on T cell response, IL-19 knockout mice increased production of inflammatory cytokines. These results show that IL-19 suppressed hapten-dependent skin inflammation in the elicitation phase of CHS.
Macrophages are pivotal cells in the innate immune system. During the pathogenesis of inflammatory diseases, macrophage organize the first host reaction to tissue invasion.1) Macrophages activated by effector T cells, as well as directly stimulated by infected pathogens, function in the pathogenesis of some kinds of inflammatory diseases.2) Allergic contact dermatitis (ACD) is an inflammatory disease of the skin.3) ACD is induced by frequent exposure to foreign substances such as metals, cosmetics, drugs and plant materials.4) In the pathogenesis of ACD, many types of immune cells, including keratinocytes, macrophages, T cells, and B cells, play important roles in the initiation and termination mechanisms of ACD.5) Contact hypersensitivity (CHS) as a mouse model of ACD has been used for two decades. CHS consists of two distinct parts.6) The first is a sensitization part, which is primed by the application of a hapten to the abdominal epidermis. Thereafter, the hapten-carrier complex is taken up by dermal dendritic cells and Langerhans cells. Langerhans cells can move from the epidermis to the draining lymph nodes, where the hapten-carrier-major histocompatibility complex (MHC) complex is presented to naïve T cells. Proliferated effector T cells move out of the draining lymph nodes to the blood. The second part, called the elicitation phase, is initiated after re-sensitization of the epidermis to the same hapten. Effector T cells produce cytokines, such as interferon (IFN)γ and interleukin (IL)-17, which mediate further cellular infiltration and the characteristic edema associated with it.7)
We previously generated IL-19 deficient (IL-19 knock out (KO)) mice that demonstrate, in vivo, the importance of inflammatory bowel disease and, in vitro, the significance of macrophage function.8–10) IL-19, IL-20, IL-22, IL-24, and IL-26 belong to the same IL-10 family of interleukins. IL-19 is expressed in immune cells, such as granulocyte macrophage colony-stimulating factor (GM-CSF)-stimulated or lipopolysaccharide (LPS)-stimulated and naive monocytes,11) activated and naive B cells,12) and non-immune cells such as epithelial cells and keratinocytes.13) IL-19 signaling is mediated by the IL-20 receptor, a heterodimer receptor composed of an IL-20 receptor α subunit (IL-20Rα) and an IL-20 receptor β subunit (IL-20Rβ).14) IL-20Rα and IL-20Rβ are expressed in non-immune cells such as keratinocytes. In contrast, only IL-20Rβ is expressed in immune cells including natural killer (NK) cells, monocytes, B cells, and T cells.13) IL-19 is distinctly correlated with the progress of the T-helper 2 (Th2) phenotype, including the pathogenesis of asthma.15,16) IL-19 serum levels are increased in patients with asthma, corresponding to the levels of Th2 mediators, including IL-4 and IL-13.15) Moreover, IL-19 is related to the pathophysiology of psoriasis, a Th1-mediated epidermal disease.17–19) The psoriatic epidermis has high IL-19 expression,19) and IL-19 released by keratinocytes upregulates the production of keratinocyte growth factor from CD8+ T cells, which form a positive feedback loop that exacerbates psoriasis.18) Thus, IL-19 regulates the development of psoriasis through the facilitation of the Th1 response. However, the involvement of IL-19 in ACD and the CHS response is not well understood. IL-19 is expressed in keratinocytes, macrophages, and B cells. In addition, IL-19 affects T cell function. Based on this evidence, we hypothesized that IL-19 may mediate a crucial function in CHS. This is the first report that shows the striking finding of IL-19 in the activation of inflammatory responses and in the control of IL-17-mediated epidermal immune reactions in an in vivo CHS model.
MATERIALS AND METHODS
MiceWe used IL-19KO mice on a BALB/c background.10) Heterozygous mice were crossed to produce IL-19KO and wild-type (WT) control mice. All mice used were 8–15 weeks old. All animal protocols were approved by the Osaka Prefecture University Animal Care and Committee.
Hapten-Induced Contact HypersensitivityMice were sensitized by applying 25 µL of 0.5% 1-fluoro-2,4-dinitrofluorobenzene (DNFB, Sigma-Aldrich, St. Louis, MO, U.S.A.) in vehicle solution (acetone : olive oil 3 : 1, v/v) to shaved abdominal skin. Five days after sensitization, the mice were challenged by painting 20 µL of 0.1% DNFB on their ears. Mice were sensitized by applying 150 µL of 0.5% fluorescein isothiocyanate isomer I (FITC, Sigma-Aldrich) in vehicle solution (acetone : dibutyl phthalate 1 : 1, v/v) to shaved abdominal skin. Five days after sensitization, the mice were challenged by applying 20 µL of 0.5% FITC on their ears. Ear swelling was measured with a micrometer 24 and 48 h after the challenge. Ear swelling was evaluated as the thickness of the ear before and after the challenge.
Histological AnalysisMouse ears were routinely stained with hematoxyline and eosin (HE) according to the manufacturer’s instructions. Mast cell infiltration in the ears was stained with toluidine blue.10)
Immunofluorescent StainingFrozen sections of the ears were used. Immunofluorescent staining was performed as described previously20) with some modifications.21) We used a rat anti-mouse F4/80 antibody (AbD Serotec, Oxford, U.K., CI:A3-1) to detect macrophages and a rat anti-mouse Gr-1 antibody (AbD Serotec, RB6-8C5) to detect neutrophils. Dendritic cells were detected using a hamster anti-mouse CD11c Alexa Fluor488 antibody (AbD Serotec). CD4+ T cells and CD8+ T cells were detected by Alexa Fluor 488 anti-mouse CD4 and Alexa Fluor 488 anti-mouse CD8a antibodies (BioLegend, San Diego, CA, U.S.A.). The double-positive immunoreactivities of IL-19 (rabbit anti-IL-19 polyclonal antibody; Abcam plc, Cambridge, U.K.) were detected using confocal images collected using a laser-scanning microscope.
Quantitative RT-PCRQuantitative real-time PCR was used to measure IL-19 and IFNγ mRNA expression in the ears and lymph nodes and was performed as described previously.22) The primers for IL-19, IFNγ, and IL-17 were 5′-GCC ATG CAA ACT AAG GAC ACC-3′ and 5′-TTG GTC ATG CAG CAC ACA TC-3′, 5′-TAC ACA CTG CAT CTT GGC TTT G-3′ and 5′-CTT CCA CAT CTA TGC CAC TTG AG-3′, and 5′-GCT CCA GAA GGC CCT CAG A-3′ and 5′-AGC TTT CCC TCC GCA TTG A-3′, respectively. Hypoxanthine phosphoribosyltransferase (HPRT) mRNA was used to normalize mRNA expression.
ImmunohistochemistryImmunohistochemical staining of frozen sections of the ears was performed as described previously23) with some modifications.24) The immunoreactivity of IL-19 was detected using the 3,3′-diaminobenzidine (DAB) system.
Cell Isolation and Cytokine ProductionCells were isolated from the inguinal and axillary lymph nodes taken from mice sensitized with 0.5% DNFB on their shaved abdominal skin 5 d previously. The cells were cultured with or without 2,4-dinitrobenzenesulfonic acid dehydrate (DNBS; Wako Pure Chemical Industries, Ltd., Osaka, Japan) (as the soluble form of DNFB) for 72 h. The concentration of the cytokines was determined by MAGPIX (Bio-Rad, Hercules, CA, U.S.A.).
Splenocytes were isolated from the spleen and were activated in T-cell activation plates as described previously.9) After 48 h, the concentration of IL-17 was determined using an enzyme-linked immunosorbent assay (ELISA).
Flow CytometryPreparation of single-cell suspensions was performed as described previously.25) The ears were collected, cut into small pieces, and digested with 0.25 mg/mL Liberase™ (Roch Diagnostics GmbH, Mannheim Germany) and 12.5 µg/mL deoxyribonuclease (DNase) I (Sigma-Aldrich) at 37°C for 1 h. Cells from the ears were treated with 0.2 µg/mL Fc blocker (anti-mouse CD16/CD32 antibody (2.4G2 clone); TONBO Biosciences, San Diego, CA, U.S.A.) and reacted with an anti-mouse CD8a-FITC antibody (Miltenyi Biotec, Auburn, CA, U.S.A.). Flow cytometry was performed using an S3e™ cell sorter (Bio-Rad).
Statistical AnalysesError bars indicate the standard error of the mean (S.E.M.) Statistical significance was determined by a one-way ANOVA for non-repeated measures to detect differences among the 4 groups. The differences between groups were determined using the Tukey–Kramer test. The statistical significance of the parametric data was evaluated using the two-tailed Student’s t-test to detect differences between 2 groups. A p-value of less than 0.05 was considered statistically significant.
RESULTS
The DNFB-Induced CHS Response Was Increased in IL-19KO MiceWe quantified the phenotype of IL-19KO mice to the hapten-challenged CHS responses by measuring ear thickness. IL-19KO mice showed severe ear swelling compared with WT mice at 24 and 48 h after the DNFB challenge (Fig. 1A left panel). In contrast, ear swelling was almost the same between the knockout and WT mice after the FITC challenge (Fig. 1A right panel). Ear tissue from IL-19KO mice revealed infiltrated cells and edema 48 h after the DNFB challenge (Fig. 1B). Because IL-19KO mice showed severe ear swelling, we focus on the CHS responses by the DNFB challenge.
We checked for the infiltration of macrophages, neutrophils, mast cells, and dendritic cells in the ears. As shown in Fig. 2A, the ears of IL-19KO mice had a high population of Gr-1+ cells. However, the recruitment of macrophages (Fig. 2A), mast cells (Fig. 2B), and dendritic cells (data not shown) in the ears of IL-19KO mice (evaluated using an anti-F4/80 antibody, toluidine blue staining, and an anti-CD11c antibody) were at the same level as those in the ears of WT mice. These findings show that IL-19 deficiency exacerbated the DNFB challenge-induced CHS response in mice.
IL-19 Expression Is Increased in the EarAs shown in Fig. 3A, the DNFB challenge in WT mice induced IL-19 mRNA expression in the ear. To confirm the results of the mRNA analysis, we conducted immunohistochemistry on the ear tissue for IL-19 expression. As shown in Fig. 3B, the expression of IL-19 was absent in the ears of WT mice that were not challenged with DNFB. There was a marked increase in the level of IL-19 in the border between the epidermis and dermis, as well as in the dermis of the ear, after the DNFB challenge. To identify which types of cells produce IL-19, we stained the tissues with F4/80, Gr-1 CD11c, CD4, and CD8a fluorescent immune cell marker antibodies. As shown in Fig. 3C, IL-19 was fully co-localized with F4/80 and partially co-localized with Gr-1. IL-19 was not co-localized with CD11c, CD4, or CD8a (data not shown).
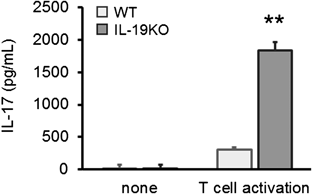
Hapten-Specific T Cell Priming in IL-19KO Mice Is Comparable to That in WT MiceTo address the mechanistic details underlying the IL-19-mediated DNFB challenge-induced CHS responses in mice, we examined the response of antigen-specific T cells. We used regional lymph nodes harvested 5 d after DNFB sensitization and evaluated the response of T cells to DNBS, a water-soluble reagent having the same antigenicity as DNFB. As shown in Fig. 4, compared with WT mice and in response to DNBS, T cells from IL-19KO mice induced markedly high production of IL-17 and IL-6, but not IFNγ and IL-4.
IFNγ and IL-17 Expression in the EarIFNγ and an accumulation of Th1/Tc1 cells were observed at the ear in this DNFB-induced CHS response. In addition to confirming increased IFNγ expression, we examined whether IL-17 expression was increased in the ear. As shown in Fig. 5, the DNFB challenge in WT mice showed expression of IFNγ and IL-17 mRNA in the ear. IFNγ expression, but not IL-17 expression, was significantly increased in DNFB-challenged WT mice. These results suggest that IFNγ is a key cytokine and that IL-17 is not a key cytokine but does exacerbate inflammation in this CHS response model.
IL-19 Function Is Linked to the IL-17-Mediated, but Not the IFNγ-Mediated PathwayTo investigate the hypothesis that the increased CHS response in IL-19KO mice was correlated with the enhancement of a hypersensitivity, we examined the levels of IL-17 in T cells and found that splenic T cells from IL-19KO mice produced increased amounts of IL-17 (Fig. 6).
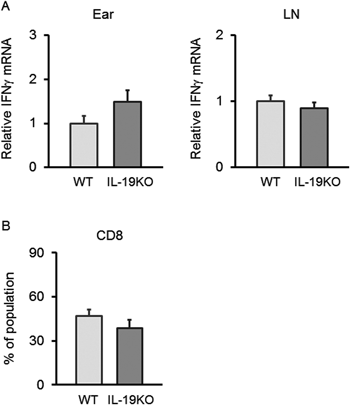
The CHS response to a DNFB challenge is mediated by the Th1 cytokine IFNγ. Tc1 cells, as well as Th1 cells, secrete IFNγ. We already showed that, in response to DNBS treatment, T cells from IL-19KO mice produced the same levels of IFNγ as T cells from WT mice (Fig. 4 lower left panel). To further refute the hypothesis that the increased CHS response in IL-19KO mice is correlated with increased IFNγ, we confirmed IFNγ levels in the tissues using qPCR and flow cytometry analysis. These procedures showed the same IFNγ mRNA levels in the ear and lymph nodes, and the same population of CD8+ T cells in the ears of WT mice as that of IL-19KO mice with a CHS response to a DNFB challenge (Fig. 7). Our findings indicate that IL-19 can regulate the IL-17-mediated response.
Increased Inflammatory Responses in IL-19KO MiceWe next examined whether IL-19 expression changed during the sensitization phase. As shown in Fig. 8, the sensitization with DNFB in WT mice showed no further induction of IL-19 mRNA in the lymph nodes. IL-19 expression was present in the lymph nodes of WT mice without DNFB sensitization. In contrast, IL-19 expression was absent in the ears of WT mice without DNFB sensitization (Fig. 3A). To investigate the role of IL-19 deficiency in the sensitization phase, we cultured cells from the lymph nodes of IL-19KO mice sensitized with DNFB. As shown in Fig. 9, cells derived from the lymph nodes of IL-19KO mice released increased levels of IL-1β, IL-12, tumor necrosis factor (TNF)-α (not statistically significant), GM-CSF, C-C motif chemokine 5 (CCL5), chemokine (C-X-C motif) ligand 1 (CXCL1), macrophage inflammatory protein-1α (MIP-1α), and MIP-1β. Thus, IL-19KO mice showed enhanced inflammatory responses at the sensitization phase.
DISCUSSION
This study is the first to show that IL-19 regulates the DNFB-induced CHS response. We showed that IL-19 deficiency exacerbated the CHS response to a DNFB challenge, whereas there was no marked difference in the CHS response to a FITC challenge. One of the most striking findings presented here is the strong inflammatory phenotype in DNFB-challenged IL-19KO mice and the complete absence of this phenomenon in FITC-mediated CHS. The functional explanation for the differential inflammatory CHS to a FITC versus a DNFB challenge is that the response to a DNFB challenge is mediated by Th1 IFNγ-inducing haptens,26) whereas the response to a FITC challenge is mediated by a Th2 response.27) IL-19 is correlated to the progress of Th2-type diseases such as asthma.15,16) We believe that immune cells and signaling within the immunological network are different depending on the type of diseases; this includes CHS responses and asthma. These results suggest that IL-19 does not contribute to FITC-mediated Th2 responses in skin inflammation, unlike respiratory inflammation.
Our results also show that IL-19 signaling predicts a crucial function in a development of hapten-specific host reactions in the epidermis by regulating IL-17-producing cells such as Th17 cells. IL-17 released at regions of hapten exposure has been shown to play a stimulatory role in the sensitization phase28) and the effector part.29) IL-17 is expressed by γδT cells, as well as Th17 cells, in the skin. Jiang et al. reported that γδT cells play an important role in the CHS response by producing IL-17, which promotes neutrophil infiltration.30) It is likely, therefore, that IL-19 suppresses the activation of IL-17-producing cells including Th17 cells or γδT cells. Another possibility is that IL-19 may suppress the differentiation of naïve T cells to Th17 cells or γδT cells. Nonetheless, the immunological collaboration that seems to occur between IL-19 and IL-17 is an important interaction in inflammation of the skin. IL-19 affects the production of IL-17. Namely, IL-19 inhibits the production of IL-17A and promoted a Staphylococcus aureus-induced skin infection,31) which supports our data. We also showed that IL-17 production increased in IL-19KO mice with 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis. In particular, lymph node cells in IL-19KO mice with TNBS-induced colitis have increased levels of IL-17.9) Mice with TNBS-mediated colitis have a marked Th1 response.32) In other models of colitis, cells derived from the lymph nodes of IL-19KO mice with oxazolone-induced colitis released the same levels of IL-17 upon activation with anti-CD3 and anti-CD28 antibodies compared with WT mice (unpublished data). Mice with oxazolone-mediated colitis are important for the research of a Th2 phenotype.33,34) These data show that IL-17-mediated responses are enhanced in the absence of IL-19 signaling. We showed that the number of Gr-1-positive cells in the ears of IL-19KO mice increased. How does IL-19 affect Gr-1-positive cells? Many kinds of chemokines with a CHS response were altered in the lymph nodes and skin. Therefore, it seems that one or more chemokines for Gr-1-positive cells, such as CXCL1, were increased by an IL-19 deficiency. One possible explanation is that increased IL-17 through IL-19 deficiency enhanced the infiltration of Gr-1-positive cells in the ear because IL-17 produces some chemokines and elicits skin inflammation.
Interestingly, there is a possible relationship between IL-17 and the FITC-induced CHS response. Cowden JM et al. previously reported that a reduced CHS response induced by FITC was accompanied by decreased IL-17 levels.35) Therefore, the interactions among IL-19, IL-17, and the Th2 response may be an interesting topic of future research.
We showed that IL-19KO mice failed to increase IFNγ production in the ears, lymph nodes, and T cells in the lymph nodes and to alter the population of CD8+ T cells, which are the main sources of INFγ in Th1-type CHS response in the ear.7) NK cells and NKT cells, as sources of INF-γ, contribute to the CHS response.36) To date, no studies have investigated the possible involvement and effect of IL-19 signaling on NK cells and NKT cells. Further study is needed to determine the role of IL-19 in NK cells and NKT cells. It is unlikely that IL-19 is related to the Th1 response in skin inflammation.
The population of CD4+ T cells includes not only Th1, Th2, and Th17 cells contributing to inflammation but also regulatory T cells, which suppress the immune response using cytokines such as IL-10 and TGF-β. Our data show similar levels of IL-10 and TGF-β mRNA in the lymph nodes of IL-19KO mice with a DNFB challenge-induced CHS response compared with WT mice (data not shown). This shows that IL-19 is directly involved in IL-17-producing cells.
We found that IL-19 is not expressed in the ears of WT mice with or without DNFB sensitization. A DNFB challenge is needed to induce IL-19 expression in the ear. In contrast, IL-19 is expressed in the lymph nodes of WT mice without DNFB sensitization. This is the reason that many types of inflammatory cytokines were increased in the cells derived from the lymph nodes of IL-19KO mice in the sensitization phase. In skin inflammation, several types of cells are candidates as the source of IL-19 production. Our data indicate that F4/80-positive cells fully produced IL-19 and Gr-1-positive cells only partially produced IL-19 during the elicitation part of the CHS response. As far as we know, there is no report that neutrophils produce IL-19. Therefore, it is likely that F4/80-positive and Gr-1-positive macrophages produce IL-19 in the ear during the CHS response. Our results showed that IL-19 has dual functions in the CHS response, regulating IL-17-producing cells and controlling inflammatory cytokines. Whereas some roles affect the development of the CHS response, other roles may be more evident in several stage of the CHS response.
In this study, we addressed the function of IL-19 in skin inflammation. In conclusion, these results indicate that IL-19 influenced IL-17-producing cells under the Th1-mediated immune response and that there is a general, increased tendency of immune cells to produce cytokines and chemokines. Collectively, our results clarify the regulatory mechanisms of IL-19 in vivo. IL-19 is a new mediator with anti-inflammatory effects.
Acknowledgment
This work was supported by a Grant-in-Aid for Scientific Research (B) 25292174 (Y.T.A.).
Conflict of Interest
The authors declare no conflict of interest.
REFERENCES
- 1) Steinfelder S, O’Regan NL, Hartmann S. Diplomatic assistance: can helminth-modulated macrophages act as treatment for inflammatory disease? PLOS Pathog., 12, e1005480 (2016).
- 2) Liang Y, Kwota Z, Sun J. Intrahepatic regulation of antiviral T cell responses at initial stages of viral infection. Int. Immunopharmacol., 39, 106–112 (2016).
- 3) Maeda N, Yamada C, Takahashi A, Kuroki K, Maenaka K. Therapeutic application of human leukocyte antigen-G1 improves atopic dermatitis-like skin lesions in mice. Int. Immunopharmacol., 50, 202–207 (2017).
- 4) Martins JD, Maciel EA, Silva A, Ferreira I, Ricardo F, Domingues P, Neves BM, Domingues MR, Cruz MT. Phospholipidomic profile variation on THP-1 cells exposed to skin or respiratory sensitizers and respiratory irritant. J. Cell. Physiol., 231, 2639–2651 (2016).
- 5) Li X, Wang X, Jiang H, Zhang G, Tan R, Sun Y, Wu X, Tan R, Xu Q. Herpetol ameliorates allergic contact dermatitis through regulating T-lymphocytes. Int. Immunopharmacol., 40, 131–138 (2016).
- 6) Christensen AD, Haase C. Immunological mechanisms of contact hypersensitivity in mice. APMIS, 120, 1–27 (2012).
- 7) Xu H, DiIulio NA, Fairchild RL. T cell populations primed by hapten sensitization in contact sensitivity are distinguished by polarized patterns of cytokine production: interferon gamma-producing (Tc1) effector CD8+ T cells and interleukin (Il) 4/Il-10-producing (Th2) negative regulatory CD4+ T cells. J. Exp. Med., 183, 1001–1012 (1996).
- 8) Azuma YT, Matsuo Y, Kuwamura M, Yancopoulos GD, Valenzuela DM, Murphy AJ, Nakajima H, Karow M, Takeuchi T. Interleukin-19 protects mice from innate-mediated colonic inflammation. Inflamm. Bowel Dis., 16, 1017–1028 (2010).
- 9) Matsuo Y, Azuma YT, Kuwamura M, Kuramoto N, Nishiyama K, Yoshida N, Ikeda Y, Fujimoto Y, Nakajima H, Takeuchi T. Interleukin 19 reduces inflammation in chemically induced experimental colitis. Int. Immunopharmacol., 29, 468–475 (2015).
- 10) Fujimoto Y, Azuma YT, Matsuo Y, Kuwamura M, Kuramoto N, Miki M, Azuma N, Teramoto M, Nishiyama K, Izawa T, Nakajima H, Takeuchi T. Interleukin-19 contributes as a protective factor in experimental Th2-mediated colitis. Naunyn Schmiedebergs Arch. Pharmacol., 390, 261–268 (2017).
- 11) Gallagher G, Dickensheets H, Eskdale J, Izotova LS, Mirochnitchenko OV, Peat JD, Vazquez N, Pestka S, Donnelly RP, Kotenko SV. Cloning, expression and initial characterization of interleukin-19 (IL-19), a novel homologue of human interleukin-10 (IL-10). Genes Immun., 1, 442–450 (2000).
- 12) Wolk K, Kunz S, Asadullah K, Sabat R. Cutting edge: immune cells as sources and targets of the IL-10 family members? J. Immunol., 168, 5397–5402 (2002).
- 13) Kunz S, Wolk K, Witte E, Witte K, Doecke WD, Volk HD, Sterry W, Asadullah K, Sabat R. Interleukin (IL)-19, IL-20 and IL-24 are produced by and act on keratinocytes and are distinct from classical ILs. Exp. Dermatol., 15, 991–1004 (2006).
- 14) Azuma YT, Matsuo Y, Nakajima H, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Takeuchi T. Interleukin-19 is a negative regulator of innate immunity and critical for colonic protection. J. Pharmacol. Sci., 115, 105–111 (2011).
- 15) Liao SC, Cheng YC, Wang YC, Wang CW, Yang SM, Yu CK, Shieh CC, Cheng KC, Lee MF, Chiang SR, Shieh JM, Chang MS. IL-19 induced Th2 cytokines and was up-regulated in asthma patients. J. Immunol., 173, 6712–6718 (2004).
- 16) Gallagher G, Eskdale J, Jordan W, Peat J, Campbell J, Boniotto M, Lennon GP, Dickensheets H, Donnelly RP. Human interleukin-19 and its receptor: a potential role in the induction of Th2 responses. Int. Immunopharmacol., 4, 615–626 (2004).
- 17) Witte E, Kokolakis G, Witte K, Philipp S, Doecke WD, Babel N, Wittig BM, Warszawska K, Kurek A, Erdmann-Keding M, Kunz S, Asadullah K, Kadin ME, Volk HD, Sterry W, Wolk K, Sabat R. IL-19 is a component of the pathogenetic IL-23/IL-17 cascade in psoriasis. J. Invest. Dermatol., 134, 2757–2767 (2014).
- 18) Li HH, Lin YC, Chen PJ, Hsiao CH, Lee JY, Chen WC, Tzung TY, Wu JC, Chang MS. Interleukin-19 upregulates keratinocyte growth factor and is associated with psoriasis. Br. J. Dermatol., 153, 591–595 (2005).
- 19) Otkjaer K, Kragballe K, Funding AT, Clausen JT, Noerby PL, Steiniche T, Iversen L. The dynamics of gene expression of interleukin-19 and interleukin-20 and their receptors in psoriasis. Br. J. Dermatol., 153, 911–918 (2005).
- 20) Azuma YT, Hagi K, Shintani N, Kuwamura M, Nakajima H, Hashimoto H, Baba A, Takeuchi T. PACAP provides colonic protection against dextran sodium sulfate induced colitis. J. Cell. Physiol., 216, 111–119 (2008).
- 21) Azuma YT, Hayashi S, Nishiyama K, Kita S, Mukai K, Nakajima H, Iwamoto T, Takeuchi T. Na+/Ca2+ exchanger-heterozygote knockout mice display increased relaxation in gastric fundus and accelerated gastric transit in vivo. Neurogastroenterol. Motil., 28, 827–836 (2016).
- 22) Azuma YT, Nishiyama K, Matsuo Y, Kuwamura M, Morioka A, Nakajima H, Takeuchi T. PPARα contributes to colonic protection in mice with DSS-induced colitis. Int. Immunopharmacol., 10, 1261–1267 (2010).
- 23) Kinjo T, Azuma YT, Nishiyama K, Fujimoto Y, Miki M, Kuramoto N. Intestinal IL-17 expression in canine inflammatory bowel disease. Int. J. Vet. Health Sci. Res., (2018), in press.
- 24) Fujimoto Y, Tsuneyama K, Kuramoto N, Hayashi S, Yoshida N, Morioka A, Teramoto M, Nakajima H, Takeuchi T, Azuma YT. Exacerbated experimental pancreatitis in interleukin-19 knockout mice. Glob. Drugs Therap., 2, 1–5 (2017).
- 25) Broggi A, Cigni C, Zanoni I, Granucci F. Preparation of single-cell suspensions for cytofluorimetric analysis from different mouse skin regions. J. Vis. Exp., 110, e52589 (2016).
- 26) Nishimura K, Aoyanagi N, Uchino E, Itabashi Y. Influence of hot spring water on fatty acid composition of skin surface lipids in hairless mouse model of atopic dermatitis. Biol. Pharm. Bull., 39, 1718–1722 (2016).
- 27) Matsuoka T, Kurohane K, Suzuki W, Ogawa E, Kobayashi K, Imai Y. Dibutyl maleate and dibutyl fumarate enhance contact sensitization to fluorescein isothiocyanate in mice. Biol. Pharm. Bull., 39, 272–277 (2016).
- 28) Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, Iwakura Y. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity, 17, 375–387 (2002).
- 29) He D, Wu L, Kim HK, Li H, Elmets CA, Xu H. IL-17 and IFN-gamma mediate the elicitation of contact hypersensitivity responses by different mechanisms and both are required for optimal responses. J. Immunol., 183, 1463–1470 (2009).
- 30) Jiang X, Park CO, Geddes Sweeney J, Yoo MJ, Gaide O, Kupper TS. Dermal γδ T cells do not freely re-circulate out of skin and produce IL-17 to promote neutrophil infiltration during primary contact hypersensitivity. PLOS ONE, 12, e0169397 (2017).
- 31) Myles IA, Fontecilla NM, Valdez PA, Vithayathil PJ, Naik S, Belkaid Y, Ouyang W, Datta SK. Signaling via the IL-20 receptor inhibits cutaneous production of IL-1β and IL-17A to promote infection with methicillin-resistant Staphylococcus aureus. Nat. Immunol., 14, 804–811 (2013).
- 32) Kitani A, Fuss IJ, Nakamura K, Schwartz OM, Usui T, Strober W. Treatment of experimental (trinitrobenzene sulfonic acid) colitis by intranasal administration of transforming growth factor (TGF)-beta1 plasmid: TGF-beta1-mediated suppression of T helper cell type 1 response occurs by interleukin (IL)-10 induction and IL-12 receptor beta2 chain downregulation. J. Exp. Med., 192, 41–52 (2000).
- 33) Heller F, Fuss IJ, Nieuwenhuis EE, Blumberg RS, Strober W. Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity, 17, 629–638 (2002).
- 34) Boirivant M, Fuss IJ, Chu A, Strober W. Oxazolone colitis: A murine model of T helper cell type 2 colitis treatable with antibodies to interleukin 4. J. Exp. Med., 188, 1929–1939 (1998).
- 35) Cowden JM, Zhang M, Dunford PJ, Thurmond RL. The histamine H4 receptor mediates inflammation and pruritus in Th2-dependent dermal inflammation. J. Invest. Dermatol., 130, 1023–1033 (2010).
- 36) Shimizuhira C, Otsuka A, Honda T, Kitoh A, Egawa G, Nakajima S, Nakashima C, Watarai H, Miyachi Y, Kabashima K. Natural killer T cells are essential for the development of contact hypersensitivity in BALB/c mice. J. Invest. Dermatol., 134, 2709–2718 (2014).