2018 Volume 41 Issue 3 Pages 350-359
2018 Volume 41 Issue 3 Pages 350-359
Isatin (1H-indole-2,3-dione) and many of its derivatives are reported to have pharmacological properties. In this study, we report the synthesis and biological activity of a new class of N-alkyl-isatin-3-iminobenzoic acid derivatives prepared via the condensation of N-alkyl isatin with 4-aminobenzoic acid by conventional, microwave, and ultrasonic methods. Microwave irradiation yielded the products in a shorter reaction time with higher yields and purities. The compounds were screened in zebrafish embryos, and also in three human cancer cell lines (MCF7, HepG2, and Jurkat) and one normal human cell line i.e., human foreskin cell line (HFF-1). Two compounds (3c, 3f) were found to be highly effective against hematopoiesis in live zebrafish embryo at 10 µM concentration. The developmental stage-dependent treatment indicated that these compounds interfered with the differentiation of hemangioblasts to hematopoietic cells in zebrafish embryos. The comparative screening of semaxanib (SU5416) (a known isatin derivatives), to compounds synthesized in this study, revealed the contrasting effects of these two classes of isatin derivatives on zebrafish hematopoiesis. Most of the N-alkyl-isatin-3-iminobenzoic acid derivatives were toxic on cancer and non-cancer tested human cells lines, however, the compounds 3c and 3f specifically affected the cell viability of Jurkat cells (human hematological cell line) with least IC50 values of 16.5 and 7.8 µM. The structure–activity relationship (SAR) analysis indicated that the substitution pattern of the isatin at the 5-position was vital for activity. The in vivo and in vitro biological activities of these compounds suggested their potential use as pharmaceutical compounds for human leukemia treatment.
Leukemia, the cancer of the blood or bone marrow, is characterized by an abnormal increase of immature white blood cells. The Middle East has the highest mortality rate from leukemia in the world and, in a comparison of countries worldwide, Iraq ranked first, with a mortality rate of 6.85.1) Similarly, leukemia is the leading cancer among children in Saudi Arabia and accounted for 38.8% of childhood cancers in 2013. Furthermore, leukemia accounts for 5.9% of all newly diagnosed cancers in Saudi Arabia and was the third most common cancer among males and the sixth most common cancer among females in 2013.2) The high mortality rate associated with leukemia in the Middle East is very alarming and has necessitated to design and synthesis novel and effective chemotherapeutic agents. Isatin (1H-indole-2,3-dione) and many isatin derivatives have been reported to possess diverse biological activities.3–5) In a previous study, we reported the synthesis and anticancer activity of isatin hydrazide-hydrazone derivatives with valproic acid (VPA).6) These derivatives have shown activity in cancer cell lines, but were particularly effective in the Jurkat cell line (human hematological cell line), compared with that in human liver cancer (HepG2) cells or human embryonic kidney (HEK293) cells. Owing to the promising anticancer profile of isatin hydrazide-hydrazone derivatives in a hematological cancer cell line, we have designed six new structural derivatives of isatin-3-iminobenzoic acid and reporting the in-vitro and in-vivo biological activity. The newly synthesized compounds were first screened in zebrafish embryos in order to evaluate the developmental toxicity and bioactivity (especially the anti-hematopoietic profile) in an animal model. The results obtained from zebrafish embryos were further verified in vitro by testing the cytotoxicity of these compounds in human hematological cancer cell line (Jurakt) and two solid cancer lines i.e., HepG2 and human breast cancer (MCF7) cells. In order to assess the toxicity on normal cells, the compounds were also tested in HFF-1 (ATCC® SCRC-1041) a cell line derived from human foreskin cells.
In order to achieve the synthesis of the target compounds “3a–f” the N-alkylation of isatin is usually prepared in the presence of K2CO3 in N,N-dimethylformamide (DMF). This method is conducted for 48 h at room temperature,7) and has been modified using microwave irradiation (MW) or ultra-sonication (US). Microwave irradiation usually afforded the product in a shorter reaction time with a higher yield (Experimental, Chart 1).

i) 48 h rt, MW 20 min or 40US at 40°C; ii) 1 h reflux or MW 20 min.
Zebrafish embryos were used as an in vivo animal model system to evaluate the biological activity of these new synthesized N-alkyl-isatin-3-iminobenzoic acid derivatives. The embryos were soaked with serial dilutions (1–100 µM) of the compounds in embryo water. Each compound showed dose-dependent toxicity against the embryos. As shown in Table 1, compounds 3c, e, and f were more toxic to zebrafish embryos than were 3a, b, and d. Compound 3c was the most toxic and 100% lethality was observed in exposed embryos treated at ≥30 µM.
| S. No. | Compound ID | Toxicity in zebrafish embryos (LC50 values) (µM) | Minimum concentration at which hematopoietic defects were observed in zebrafish embryos (µM) |
|---|---|---|---|
| 1 | 3a | Not active* | Not active* |
| 2 | 3b | Not active* | Not active* |
| 3 | 3c | 28 | 20 |
| 4 | 3d | Not active* | Not active* |
| 5 | 3e | 50 | Not active* |
| 6 | 3f | 26 | 10 |
* If a compound induce require phenotype/effects at ≥100 µM, was considered as not active. All experiments were repeated three times with different batch of embryos every time and with at least 35 embryos per treatment per concentration.
The sub-lethal concentrations (10–30 µM) of compounds 3c and f induced teratogenicity and congenital malformations in exposed zebrafish embryos. The most prominent phenotype observed after the exposure of these compounds to zebrafish embryos was a lack of active circulation during exposure. Compound 3f was the most active and hindered blood circulation at ≤10 µM. The embryos that remained exposed to compound 3f were unable to restore normal circulation even up to 5 d post-fertilization. The comparative anti-hematopoietic profiles of these compounds are presented in Table 1.
In order to validate the hematopoietic defects in treated zebrafish embryos, the treated embryos were stained with O-dianisidine to visualize the erythroid cells (red blood cells). As shown in Fig. 1, the zebrafish embryos treated with 10 µM compound 3f lacked positive O-dianisidine staining in the yolk sac and in the posterior trunk, which confirmed that the lack of circulation in treated embryos resulted from the absence of erythroid cells.

Representative images of zebrafish embryos at 48 hpf processed for O-dianisidine staining. The mock (1%V/V methanol) treated embryos had normal hematopoiesis as indicated by positive O-dianisidine staining denoted by thin black arrow head, whereas the embryos exposed to compound 3f (10 µM) were completely negative of-dianisidine staining.
The zebrafish embryos were exposed to compound 3f at various developmental stages in order to identify at which developmental stage the hematopoietic process was affected. First, a batch of zebrafish embryos were exposed to compound 3f from the shield stage to 24 hours post fertilization (hpf) (before the onset of hematopoiesis) up to 48 hpf and then processed for O-dianisidine staining. Compound 3f was added only after 24 hpf to the second batch of embryos, which were exposed for an additional 24 h before being processed for erythroid staining. The third batch of embryos was exposed to compound 3f after 48 hpf (when the hematopoiesis process was almost complete) and remained exposed for 24 h (until 72 hpf). As shown in Fig. 2, the zebrafish embryos that were exposed to compound 3f from the shield stage to 24 hpf showed positive O-dianisidine staining, whereas very weak staining was observed in embryos that were exposed to 3f from 24 to 48 hpf (Fig. 2C). The zebrafish embryos that were exposed after 48 hpf did not respond to the treatment and the blood cells formed normally (Fig. 2D).

Representative images of zebrafish embryos processed treated at different times interval as indicated and then processed for O-dianisidine staining to see the formation of blood cells. A) Un treated or mock (1%V/V methanol) treated embryos had normal hematopoiesis as indicated by positive O-dianisidine staining shown by white arrow head, B) whereas the embryos which were exposed to compound 3f from shield stage until 48 hpf did not stain with O-dianisidine, C) A weaker staining was observed in those embryos which were exposed compound 3f from 24–48 hpf not before, D) The embryos which were exposed after 48 hpf to compound 3f did not respond at all and developed the blood cells normally shown by positive erythrocyte staining (white arrow).
Semaxanib (SU5416), which is an isatin derivative molecule8) was used as positive control in this study. SU5416 is a vascular endothelial growth factor (VEGF) receptor tyrosine kinase inhibitor9) and antiangiogenic activity of this compound has been reported previously,9–11) however, the hematopoietic activity of SU5614 in zebrafish embryos is largely unknown. In order to determine the effects of SU5416 on zebrafish hematopoiesis, embryos were treated with 1–10 µM SU5416 from the shield stage and processed for O-dianisidine staining at 48 hpf. The treated embryos had severe blood vessels formation defects upon treatment of SU5416 as indicated by absence of intrasomatic blood vessels (isv) in Tg(Fli-1: Enhanced Green Fluorescent Protein (EGFP)) embryos at 48 hpf (Fig. 3D), where the erythrocytes developed normally, as shown by the positive O-dianisidine staining in SU5416 treated embryos (Fig. 3, black arrow head in F). The O-dianisidine staining was observed in posterior trunk in caudal vein, which has been shown in small inset in Fig. 3F.

The representative micrograph of zebrafish embryos from Tg(Fli-1: EGFP) either mock treated (left panel) or treated with 1 µM of SU5416 from shield until 48 hpf (right panel). The control embryos had normal blood vessel formation as shown by white arrow (C), and also blood cells formed normally (E), the embryos treated with SU5416 had malformed blood vessels (D), whereas the blood cells formed normally as indicated by black arrow in F. The inset in F shows the same embryo at higher magnification showing the pooling of blood cells in caudal vein area.
The embryos from transgenic zebrafish Tg(Fli-1: EGFP)y1 12) were treated with serial dilutions of N-alkyl-isatin-3-iminobenzoic acid derivatives in order to evaluate their effects on blood vessel formation. As shown in Fig. 4, compound 3f did not hinder angiogenic blood vessel formation in treated embryos. Similarly, no alkyl-isatin-3-iminobenzoic acid derivatives were effective in blocking the angiogenic blood vessel formation in treated embryos at all the tested concentrations.

The representative micrograph of zebrafish embryos from Tg(Fli-1: EGFP)y1 line, control (A, C) or treated with compound 3f (B, D) at or before shield stage and photographed at 48 hpf. All the blood vessels (green fluorescents) including angiogenic blood vessels (intrasomatic blood vessels, isv) as indicated by white arrow formed normally in control embryos. Zebrafish embryo treated with 3f (10 µM) had no circulation and cardiac edema can be seen in bright field images (B) but the blood vessels formed normal in the treated embryos as indicated by white arrow showing normal structure of intersomatic blood vessels (isv).
The newly synthesized N-alkyl-isatin-3-iminobenzoic acid derivatives were screened via an in vitro colorimetric 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cell viability assay in three human cancer cell lines, MCF7, HepG2, and Jurkat to assess their cytotoxicity in cancer cells and also in normal cells HFF-1. The known anticancer drugs 5-fluorouracil (5-FU) and SU5416 were used as positive control to assess the comparative biological activity of N-alkyl-isatin-3-iminobenzoic acid derivatives with known anticancer molecules. As shown in Table 2 and Figs. 5–7, most of the compounds affected cell viability of cancer cell lines and showed weaker to moderate level of cytoxicity in normal human foreskin cells (Table 2, Fig. 8), however, the cytotoxicity of compounds 3c and f was much stronger in Jurkat with IC50 values (16.5, 7.8 µM, respectively) as compared with their activity in HepG2 and MCF7 cells. The cytotoxicity of compound 3c in MC7 and HepG2 was 24.14 and 37 µM, while the IC50 values of compound 3f were 34.61 and 66 µM in MCF7 and HepG2, respectively. The cytoxicity of these two compounds was very much weak in normal human foreskin cells. The compound 3c was not active (with IC50 values more than 200 µM), while the IC50 values for compound 3f was 91.53 µM (Table 2) in normal human foreskin cells (HFF-1). In comparison with compounds 3c and f, the cytotoxicity of SU5416 was very weak in tested cancer cell lines. The IC50 values of SU5416 in HepG2 and Jurkat were 112 and 90 µM, whereas in MCF7 the IC50 value of SU5416 was calculated as 41.39 µM (Table 2). The next level of cytotoxicity was found in compounds 3a and e. The IC50 values for compounds 3a and e were 14.3 and 24.3 µM, respectively in Jurkat cells. While compound 3a was not active in MCF7 and HepG2 cells and the IC50 values for compound 3e were 23.59 and 41.00 µM in MCF7 and HepG2 cells, respectively. In order to assess the cytotoxicity of these compounds in normal (non-cancers) cells, the human foreskin cell HFF-1 were treated and IC50 values were calculated. As shown in Table 2 and Fig. 8, the IC50 values of HFF-1 cells indicated that most of these compounds required very high concentration to suppress 50% survival of the normal cells as compared with their activity in cancer cells. The compound 3c which ranked second active compound in Jurkat cells with IC50 values of 16.5 µM was non active in human normal foreskin cells line (Table 2), whereas compound 3f (the most active compound against Jurkat cells) needed 91.53 µM to inhibit the 50% survival of human normal foreskin cells.
| Compounds | IC50 values µM* | |||
|---|---|---|---|---|
| MCF7 | HepG2 | Jurkat | HFF-1 | |
| 3a | NA** | NA | 14.3 | 61.29 |
| 3b | 66 | 91 | 61.4 | NA |
| 3c | 25.14 | 37 | 16.5 | NA |
| 3d | NA | NA | 28.03 | 81.66 |
| 3e | 23.59 | 41 | 24.3 | 22.8 |
| 3f | 34.61 | 66 | 7.8 | 91.53 |
| 5-FU | NA | 38 | 39.26 | 37.87 |
| SU-5416 | 41.39 | NA | 89.63 | 31.86 |
* IC50 values were calculated based on five different concentrations of the compounds and an average three biological repeats using the Origin Pro version 8.5. ** The compounds scored as not active (NA) if the IC50 values were more than 100 µM.

The line graph showing the effect of compounds on Jurkat cells line. The inhibition in cancer cell line was measured by MTT assay, and represented as % of control (untreated) cells. The average values of at least five replicates are presented.

The line graph showing the effect of compounds on HepG2 cells line. The inhibition in cancer cell line was measured by MTT assay, and represented as % of control (untreated) cells. The average values of at least five replicates are presented.

The line graph showing the effect of compounds on MCF7 cell line. The inhibition in cancer cell line was measured by MTT assay, and represented as % of control (untreated) cells. The average values of at least five replicates are presented.

The line graph showing the effect of compounds on HFF-1 cells line. The inhibition in cancer cell line was measured by MTT assay, and represented as % of control (untreated) cells. The average values of at least five replicates are presented.
The N-alkyl-isatin-3-iminobenzoic acid derivatives (3a–f) were prepared via the condensation of N-alkyl isatin with 4-aminobenzoic acid in absolute ethanol and several drops of glacial acetic acid by conventional, microwave, and ultrasonic sound methods7) (Chart 1). Microwave irradiation yielded the products in a shorter reaction time—with higher yields and purities, as indicated by spectral data.
As a prototype, the 1H-NMR spectrum of 3c (Fig. 9) measured in CDCl3 showed six resonances, located at δ 5.02 (s, integrated to two protons), δ 6.55 (d, integrated to one proton), δ 6.71–6.78 (m, integrated to three protons), δ 7.10 (d, integrated to two protons), δ 7.24–7.37 (m, integrated to five protons), and δ 8.21 (d, integrated to two protons), which were assigned to CH2 protons, proton of H-4, protons of H-5, H-6, H-7, protons of H-2″, H-6″, protons of C6H5 (H-2′, H-3′, H-4′, H-5′, and H-6′, and protons of H-3″ and H-5″, respectively. The 13C-NMR spectrum of 3c exhibited a signal up-field at δ 42.83 ppm, which was assigned to the aliphatic CH2 group. The three most down-field signals at δ 161.63, 169.84, and 178.80 ppm were attributed to C=O, C=N, and COOH, respectively, whereas the aromatic carbons appeared at δ 119.33–153.92 ppm.

Isatin (1H-indole-2,3-dione) has been reported to have diverse biological activities. Many of its derivatives have been synthesized and have been reported to possess novel pharmacological effects.13–15) In this study, we have reported the synthesis and biological activity of six N-alkyl-isatin-3-iminobenzoic acid derivatives.
Zebrafish embryos are an excellent animal model for the study of hematopoiesis, because the zebrafish embryos do not need an active circulation for up to 6–7 d post fertilization; the oxygen requirements are met from the dissolved oxygen in water and the nutrients from the yolk. The primary in vivo screening of N-alkyl-isatin-3-iminobenzoic acid derivatives in zebrafish embryos revealed that they have affected the hematopoiesis as 100% of treated embryos did not have active circulation. Two compounds (3c, f) were very effective and interfered with hematopoiesis in zebrafish embryos with IC50 values in the micromolar range (20, 10 µM, respectively).
The molecular events between developmental hematopoiesis and pathological leukemia are correlated.16–18) Zebrafish has served as a suitable model to study both of these processes.19–21) Similar to mammals, zebrafish have primitive and definitive hematopoiesis. Primitive hematopoiesis begins during the gastrula segmentation (6–18 hpf) and active circulation can be seen at approximately 24 hpf. The next round of hematopoiesis, definitive hematopoiesis, starts at the pharyngula stage from 30 hpf and completes at 48 hpf.22,23) The primitive blood cells in zebrafish embryos originate from the intermediate cell mass, which contains both vascular and hematopoietic precursors.21) In this study, the hematopoiesis defects were observed in zebrafish embryos when embryos were exposed to N-alky-isatin-3-iminobenzoic acid derivatives (especially 3c, f) during the gastrula segmentation stage (6–18 hpf). In order to determine whether these compounds interfered with blood cell formation process (effecting the morphogenesis of blood cells) or destroyed the blood cells, after blood cells have been formed (cytotoxic towards blood cells) a time-dependent experiment was conducted in which zebrafish embryos were exposed to compound 3f either before or after the onset of primary and definitive hematopoiesis. As shown in Fig. 3, the time-dependent treatment of zebrafish embryos revealed that N-alky-isatin-3-iminobenzoic acid derivatives turned out to be most effective inhibitor of the hematopoietic process in zebrafish embryos. The O-dianisidine positive cells (erythrocytes) were not observed in zebrafish embryos when they were exposed to compound 3f before the onset of hematopoiesis, i.e., before the gastrula segmentation stage (6–18 hpf), whereas O-dianisidine positive cells were present in zebrafish embryos when they were exposed after 48 hpf. The formation of blood vessels and blood cells are interconnected developmental processes. The hematopoietic cells (the cells that will give rise to different blood cell lineage) and endothelial cells (the cells that will give rise to blood vessels) share a common progenitor, which is called the hemangioblast.24) In this study, it is quite clear that N-alkyl-isatin-3-iminobenzoic acid derivatives have affected the differentiation of hemangioblasts to hematopoietic cells, but not the endothelial cells. Undeveloped or malformed blood vessels could also be a reason for ineffective circulation, and hence, in order to address this point, the development of blood vessels was also checked in N-alkyl-isatin-3-iminobenzoic acid derivative-treated zebrafish embryos. The embryos from Tg(Fli-1: EGFP)y1 had normal vasculature, which were exposed to N-alkyl-isatin-3-iminobenzoic acid derivatives (Fig. 4), which also proved the derivatives targeted only hematopoietic cells and not the endothelial cells.
The hematopoietic activity of the N-alkyl-isatin-3-iminobenzoic acid derivatives synthesized in this study was compared with the known isatin derivative semaxanib (SU5416) Flk-1/KDR inhibitor, which is also a specific inhibitor of tyrosine kinase and interferes with angiogenesis activity by obstructing the VEGF to its receptor VEGFR2.9,10) However, the effect of SU5416 on hematopoiesis in zebrafish embryos is largely unknown. As shown in Fig. 4, zebrafish embryos treated with 1 µM SU-5416 had undeveloped/malformed vasculature, but were positive for O-dianisidine staining. As shown in Fig. 4, the zebrafish embryos treated with SU5416 had positive O-dianisidine staining localized more in the anterior trunk, mainly in the posterior cardinal vein (PCV) and the caudal vein (CV). SU5416 is a very strong antiangiogenic compound, which induces the severe malformation of blood vessels in treated zebrafish embryos, even at ≤1 µM. The positive O-dianisidine-stained cells in SU5416 embryos indicated that the blood cells had formed normally in these embryos. However, the blood cells pooled and clogged in the caudal and posterior cardinal veins and these cells failed to circulate back to the heart owing to the absence of active blood vessels and, typically, active circulation is not seen in these embryos. Previously, O-dianisidine staining of SU5416-treated zebrafish embryos has not been used to assess the status of erythrocytes. Hence, this study also reported that SU5416 did not affect hematopoiesis in zebrafish embryos. Interestingly, contrasting activity of these two classes of isatin derivatives, i.e., semaxanib (SU5416) and N-alkyl-isatin-3-iminobenzoic acid derivatives has been observed for zebrafish hematopoiesis. As observed in this study, semaxanib (SU5416), which is an isatin derivative, targeted the differentiation of hemangioblasts towards endothelial cells and had no effect on hematopoiesis; whereas compound 3f, which is an N-alkyl-isatin-3-iminobenzoic acid derivative, targeted the differentiation of hemangioblasts towards hematopoiesis, but had no effect on endothelial cells.
Newly synthesized N-alkyl-isatin-3-iminobenzoic acid derivatives showed very specific activity that interfered with hematopoiesis in live zebrafish embryos. In order to know whether these compounds could also affect the viability of hematological cancer cells, the anti-proliferative profile of these compounds were tested in Jurkat cells, which is a human leukemia cell line. The cytotoxicity of these compounds have also been checked in two solid cancer cell lines i.e., HepG2 and MCF7 cells and HFF-1. The cell viability assays have shown that most of these compounds showed promising cytotoxicity in tested cancer cells as compared to known anti-cancer drug 5-FU, however, the compounds 3c and f showed specific cytotoxicity towards Jurkat (leukemia cells) at very low IC50 values (16.5, 7.8 µM, respectively). Compounds 3c and f were more effective than the known anticancer drug 5-FU. The IC50 value of 5-FU was 39.26 µM in Jurkat cells. Moreover, the compounds 3c and f were more cytotoxic in MCF7, HepG2, and Jurkat cells compared with SU5416. The biological activity of compounds 5-FU in MCF7, HepG2 and Jurkat cells which has been observed in this study is in accordance with other published reports.6,25–29)
The compounds 3c and f showed very weak toxicity in human foreskin HFF-1 cells, the IC50 values of compound 3c in HFF-1 cells was more than 100 µM and hence designated as not active while the IC50 value for compound 3f in HFF-1 cells was 91.53 µM. The strong cytotoxicity of compounds 3c and f in Jurkat and comparatively weaker level of toxicity in HFF-1 warrants, the further studies of these two molecule for the designing and treatment of anticancer drugs to treat human leukemia.
In this study, the parent compound (isatin itself) did not alter the hematopoiesis in zebrafish embryos nor cell viability in Jurkat cells within the tested range of concentrations. However, the cytotoxicity of isatin derivatives in leukemia cell lines have been reported in other studies.30,31) However, to the best of our knowledge, we have not encountered any study that reports the action of isatin or any of its derivatives as an anti-hematopoietic molecule in live animals such as zebrafish. Hence, the compounds which are synthesized in this study are unique because they have been shown as effective anti-hematopoietic molecules in vivo (in live zebrafish embryos) and as an effective antileukemia molecule in vitro (in Jurkat cells). The specificity of these compounds towards hematopoiesis in zebrafish embryos indicated that they are highly specific inhibitors of molecular events, such as transcription factors and critical pathways, which activate blood cell formation in zebrafish and could be applied to treat leukemia in humans.
Structure–Activity Relationships (SAR)In this study, we have reported the synthesis of six N-alkyl-isatin-3-iminobenzoic acid derivatives (the target products). These compounds were designed based on the reported biological activities of isatin itself (I), semaxanib (SU5416) II, and sunitinib III, (previously known as SU11248). Beside U.S. Food and Drug Administration (FDA) approved anti-cancer drug for the treatment of renal cell carcinoma and gastric cancer, the Sunitinib is also in phase I/II clinical trial for the treatment of acute myeloid leukemia32) via inhibiting the FLT3 gene.33–35) Finally, other derivatives IV15,36), which have been reported with strong anticancer activity (Fig. 10).
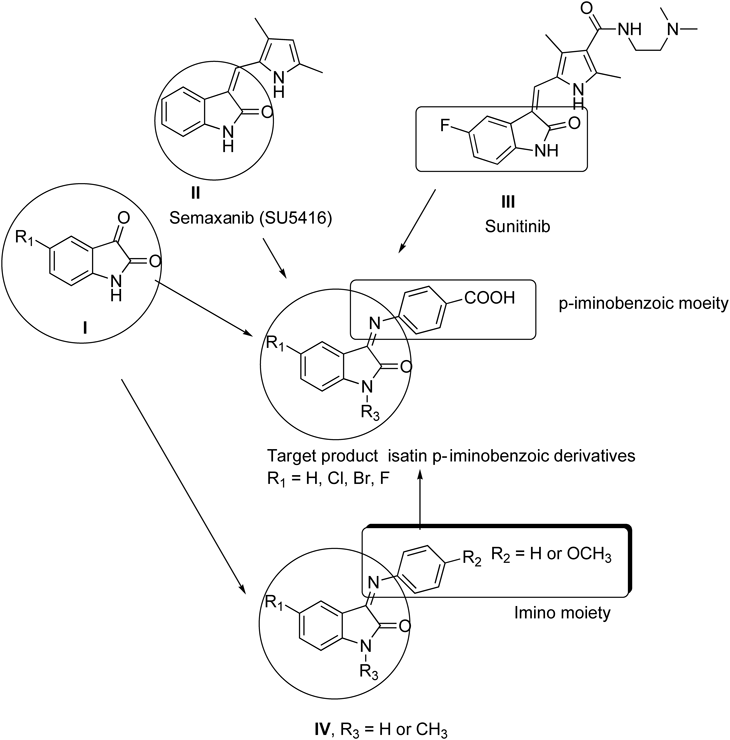
Zebrafish embryos were used as an in vivo animal model system to evaluate the biological activity of these compounds. The isatin derivatives were then screened in vitro to assess their cytotoxicity by a colorimetric MTT cell viability assay. Three human cancer cell lines, HepG2, MCF7, and Jurkat were treated with a serial dilution of the target products. The known anticancer drugs 5-FU and SU5416 were used as positive control in this study. The results from the zebrafish and the human cancer cell lines suggested that the isatin derivatives were markedly influenced by the different substituents on the isatin ring at position 5 and at the N-terminal of the isatin moiety.
The zebrafish embryos did not develop blood vessels when treated with 1 µM SU-5416, but positive O-dianisidine staining indicated that this tyrosine kinase inhibitor did not affect the blood cells formation in zebrafish embryos. In contrast, compounds 3c and f specifically inhibited blood cell formation, but did not affect the blood vessel formation in zebrafish embryos, whereas the other compounds did not affect the zebrafish embryos. This indicated that the fluorine at position 5 conferred greater activity than chlorine and bromine atoms, but, with respect to hematopoietic targeting, the benzyl group was more active than the methyl group at position 1 in the isatin moiety. The combination between fluorine and benzyl group in this series of compounds exerted a great effect on the zebrafish embryos.
The MTT cell viability assay on three cancer cell lines showed that all the tested compounds were cytotoxic in cancer cells, and cytotoxicity was better than known anticancer drug 5FU and SU-5416 against Jurkat cells. The best results were obtained again with 3c, and f whereas, 3b had the lowest efficacy; the IC50 values for 3a–f were 14.3, 61.4, 16.5, 28.0, 24.3, and 7.8 µM, respectively, compared with 39.3 and 89.6 µM for 5-FU and SU-5416, respectively in Jurkat cells. Similarly, 3c and f were more effective on MCF7 (IC50 25.14, 31.6 µM, respectively) as compared with 5-FU (301 µM) and SU-5416 (41.3 µM). The IC50 values for 3c and f were 37 and 66 µM, respectively, in HepG2 compared with 5-FU (38 µM) and SU-5416 (112 µM); 3f was similar to 5-FU, but more effective than SU-5416.
This rough SAR analysis (Fig. 10) corroborated that the substitution pattern of the isatin at the 5-position and isatin-3-iminobenzoic acid derivatives here tested were vital for activity. The most potent compounds identified were those exhibiting polar substituents (electronegative atoms) at position 5 of the isatin moiety, namely compound 3f, whereas compounds with less polar groups, namely 3a and b were less active. This suggested that electrostatic interaction (e.g., dipolar or hydrogen bond) might be critical for activity. In contrast, the N-methyl derivative (3b) showed lower activity than the N-benzyl derivative (3c), so it could be assumed that the lipophilicity of the isatin moiety at the 1-position provided a positive contribution to the activity.
We have not come across to any report about effect of Sunitinib in zebrafish embryos, however, the anti-cancer activity of Sunitinib in Jurkat cells has been reported. The cytotoxicity activity compound 3f in Jurkat cells is in accordance to thee published reports. The IC50 values of sunitinib has been reported as 637) and 2.3 µM38) in Jurkat cells, whereas the IC50 values of compound 3f was 7.8 µM in Jurkat cells in this study. The structural comparison between compound 3f and sunitinib revealed that the compound 3f is very much structurally related to sunitinib, as both of the compounds share fluorine as their moiety in isatin ring (Fig. 10) which might be center for the functional activity of these two compounds. Further, we have tested the toxicity of newly synthesized isatin derivatives in live zebrafish embryos. This assay has an advantage over other in vitro based studies that the in vivo toxicity of the compound would indicate whether the compound would be suitable to be further tested in clinical trials. The Phase III clinical trials of semaxanib for the treatment of advanced colorectal cancer were prematurely ended in February 2002 due to discouraging results.39,40) The fate of SU-5416 clearly indicates that in vivo animal studies in relevant animal models are very much necessary to assess the potential toxicity of the compounds and must be done prior to clinical trials. The zebrafish assays in this study clearly indicates that compound 3f induced hematopoietic defects at lower concentration without inducing gross teratogenicity or toxicity (Table 1). The hematopoietic defects were noticed ≤10 µM, whereas it needed more than 26 µM to induce the lethality or toxicity in zebrafish embryos. The promising bioactivity of compound 3f in Jurkat cells and zebrafish embryos warrants the design and synthesizes of similar molecules and also to check the molecular targets for example FLT3 in leukemia cells with these compounds, which is currently in progress in our lab. The promising activity means, that the compound 3f has the therapeutic potential to be developed as drug to treat leukemia after exploring the mechanism of action in leukemia cells.
All solvents used were of HPLC reagent grade. The melting points were determined with Mel-Temp apparatus and were uncorrected. Fourier transform infrared spectroscopy (FT-IR) spectra were recorded on a Nicolet 560 sepectrometer. Nuclear magnetic resonance spectra (1H- and 13C-NMR) were recorded on a JEOL 400 MHz spectrometer with chemical shift values reported in ppm (δ units) relative to an internal standard. Microwave irradiation was conducted using a multimode reactor (Synthos 3000, Anton Paar GmbH; 1400 W maximum magnetron). Reactions were performed in teflon vessels with a capacity of 10 mL. The target temperature was set and fixed during the irradiation. The settings and readings for power (W) and pressure were obtained from the instrument (J. P. Selecta, Spain; 60 Hz, 770 W). The elemental analyses were performed on a Perkin-Elmer 2400 elemental analyzer and the obtained values were within ±0.3% of the theoretical values. The follow-up of the reactions and purity assessment of the compounds were achieved by TLC on silica gel-protected aluminum sheets (GF254, Merck, Germany) and the spots were detected by exposure to a UV-lamp at λ=254 nm for a few seconds. A PerkinElmer, Inc. FT-IR Spectrometer (Spectrum 1000) was used to record FT-IR spectra of the prepared compounds as KBr pellets. The instrument is available at King Saud University, College of Science, Chemistry Department.
General Procedure for Synthesis of 1-Substituted-5-substituted-indoline-2,3-diones, 2b–fConventional Method (A)Benzyl chloride (4.1 mmol) was added dropwise to a mixture of isatin derivatives 1 (3.4 mmol) and potassium carbonate (5.1 mmol) in DMF (10 mL) at 0°C and the reaction was stirred at room temperature for 48 h. The precipitate was poured into an ice-water mixture. The resulting solid was filtered, washed with water, dried, and re-crystallized from ethanol.
Microwave Method (B)A mixture of isatin 1 (5 mmol) and potassium carbonate (8 mmol) in DMF (10 mL) was stirred for 10 min at room temperature. Alkyl halides (6 mmol; benzyl bromide for preparation of 2c–f and CH3I for the preparation of 2b) was added dropwise to the reaction mixture and then the reaction was microwave irradiated using a multimode reactor (Synthos 3000, Anton Paar GmbH, Graz, Austria) with 1400 W maximum magnetron. The vessels were heated for 5 min at 80°C and held at the same temperature for a further 5 min (400 W). A fan was used to provide cooling (5 min). The final product was dried and recrystallized from ethanol. All the spectral data obtained for the products were in good agreement with the reported data.
Ultrasonic Method (C)Benzyl chloride (4.1 mmol) was added dropwise to a mixture of isatin derivatives 1 (3.4 mmol) and potassium carbonate (5.1 mmol) in DMF (10 mL) at 0°C and the reaction was subjected to ultrasound for 4 h. The precipitate was poured into an ice-water mixture. The resulting solid was filtered, washed with water, dried, and then recrystallized from ethanol.
1-Methylisatin (2b)The product was obtained as orange red crystals in the following yields: 90% (A), 92% (B), and 87% (C); mp: 126–127°C [lit.41,42) mp 126–127°C; 73% yield]. 1H-NMR (CDCl3) δ: 3.36 (s, 3H, CH3), 6.78 (d, J=7.8 Hz, 1H, Ar), 7.12 (t, J=7.4 Hz, 1H, Ar), 7.47 (t, J=8.1 Hz, 1H, Ar), 7.61 (d, J=8.1 Hz, 1H, Ar).
1-Benzylisatin (2c)The product was obtained as orange red crystals in the following yields: 83% (A); 88% (B); 82% (C); mp: 134–136°C [lit.41,42) mp 134–136°C; 88% yield]. 1H-NMR (CDCl3) δ: 4.93 (s, 2H, C6H5CH2), 6.77 (d, J=7.7 Hz, 1H, Ar), 7.08 (t, J=7.4 Hz, 1H, Ar), 7.33 (s, 5H, Ar), 7.47 (t, J=8.1, 1H), 7.61 (d, J=8.1, 1H, Ar).
1-Benzyl-5-bromoisatin (2d)The product was obtained as red orange crystals in the following yields: 98% (A); 92% (B); 94% (C); mp: 149–151°C [lit.42) mp 152–153°C; 95% yield]. 1H-NMR (CDCl3) δ: 4.91 (s, 2H, C6H5CH2), 6.69 (d, J=8.1 Hz, 1H, Ar), 7.24–7.32 (m, 5 H, Ar), 7.45 (dd, 1H, J=8.1 Hz, 2.2 Hz, Ar), 7.56 (d, 1H, J=2.2 Hz, Ar).
1-Benzyl-5-chloroisatin (2e)The product was obtained as orange crystals in the following yields: 98% (A); 92% (B); 94% (C); mp: 136°C [lit.26) mp 134°C; 65% yield]. 1H-NMR (CDCl3) δ: 4.94 (s, 2H, C6H5CH2), 6.69 (d, J=8.1 Hz, 1H, Ar), 7.2–7.34 (m, 5 H, Ar), 7.45 (dd, 1H, J=8.1, 2.3 Hz, Ar), 7.56 (d, 1H, J=2.3 Hz, Ar).
1-Benzyl-5-fluoroisatin (2f)The product was obtained as red powder, mp: 134°C; yields: 88% (A); 89% (B); 90 (C) %; 1H-NMR (CDCl3) δ: 4.92 (2H, s, C6H5CH2), 6.72 (1H, dd, J=8.79, 3.0 Hz), 7.18 (1H, dd, J=8.79, 3.0 Hz), 7.21 (1H, d, J=3.0 Hz) 7.28–7.38 (5H, m); 13C-NMR (CDCl3) δ: 42.9, 110.9, 111.3, 123.2, 123.5, 126.1, 127.0, 127.8, 132.9, 145.35. Anal. Calcd for C15H10FNO2 (255.25): C, 70.58; H, 3.95; N, 5.49. Found: C, 70.77; H, 4.05; N, 5.22.
General Procedure for Synthesis of 4-(1-Substituted-5-substituted-2-oxoindolin-3-ylideneamino)benzoic Acid (3a–f)Conventional Method (A)A mixture of isatin derivatives (2a–f) (0.01 M) and 4-amino-benzoic acid (0.01 M) in absolute ethanol (20 mL) was refluxed for 0.5 h in the presence of 2–3 drops of glacial acetic acid. After cooling, the mixture was filtered and the crude solid was recrystallized from ethanol.
Microwave Method (B)A mixture of isatin derivatives (2a–f) (0.01 M) and 4-amino-benzoic acid (0.01 M) in absolute ethanol (2 mL) was irradiated in the presence of two drops of glacial acetic acid in MW for 20 min at 600 W.
4-((2-Oxoindolin-3-ylidene)amino)benzoic Acid (3a)The product was obtained as an off-white solid, mp: 286–289°C; yield: 99% (A); 98% (B); [lit43); mp 288–290°C; yield 90%). IR (KBr, cm−1): 3500–2500 (br, OH, NH), 1725 (C=O, isatin ring), 1686 (C=O, acid), 1616 (C=N).
4-((1-Methyl-2-oxoindolin-3-ylidene)amino)benzoic Acid (3b)The product was obtained as yellow powder, mp: 186–188°C; yield %: 99 (A); 98 (B). IR (KBr, cm−1): 3422 (OH), 1702, 1659 (C=O), 1602. (C=N); 1H-NMR (CDCl3) δ: 3.36 (s, 3H, CH3), 6.55 (1H, d, J=7.35 Hz), 6.71–6.78 (3H, m), 7.10 (2H, d, J=8.7), 8.21 (2H, d, J=8.7 Hz); 13C-NMR (CDCl3) δ: 30.2, 114.3, 121.7, 125.2, 126.2, 126.7, 127.7, 133.4, 133.6, 146.1, 161.6, 169.8 (C=N), 178.8 (COOH). Anal. Calcd for C16H12N2O3 (280.08): C, 68.56; H, 4.32; N, 9.99. Found: C, 68.88; H, 4.43; N, 10.09.
4-(1-Benzyl-2-oxoindolin-3-ylideneamino)benzoic Acid (3c)The product was obtained as yellow powder, mp: 176–178°C; yield %: 99 (A); 98 (B). IR (KBr, cm−1): 3422 (OH), 1702, 1659 (C=O), 1602 (C=N); 1H-NMR (CDCl3) δ: 5.02 (2H, s, C6H5CH2), 6.55 (1H, d, J=7.35 Hz), 6.71–6.78 (3H, m), 7.10 (2H, d, J=8.7 Hz), 7.24–7.37 (5H, m, C6H5CH2), 8.21 (2H, d, J=8.7 Hz); 13C-NMR (CDCl3) δ: 42.83, 109.33, 114.29, 116.4, 121.7, 125.2, 126.2, 126.7, 127.7, 130.8, 133.4, 133.6, 146.1, 153.9, 161.6 (CO), 169.8 (C=N), 178.8 (COOH). Anal. Calcd for C22H16N2O3 (356.38): C, 74.15; H, 4.53; N, 7.86. Found: C, 74.34; H, 4.64; N, 7.99.
4-(1-Benzyl-5-bromo-2-oxoindolin-3-ylideneamino)benzoic Acid (3d)The product was obtained as yellow powder, mp: 260–262°C; yield: 54% (A); 60% (B). IR (KBr, cm−1): 3415 (OH), 1741, 1678 (C=O), 1602 (C=N); 1H-NMR (CDCl3) δ: 5.00 (2H, s, C6H5CH2), 6.55 (1H, d, J=2.19 Hz), 6.69 (1H, dd, J=8.43, 2.19 Hz), 7.09 (2H, d, J=8.43 Hz), 7.22–7.36 (6H, m, Ar), 8.23 (2H, d, J=8.43 Hz); 13C-NMR (CDCl3) δ: 43.0, 116.1, 126.1, 127.8, 130.8, 139.4, 153.9, 161.4 (CO), 169.6 (C=N), 178.9 (COOH). Anal. Calcd for C22H15BrN2O3 (434.03): C, 60.71; H, 3.47; N, 6.44. Found: C, 60.94; H, 3.65; N, 6.67.
4-(1-Benzyl-5-chloro-2-oxoindolin-3-ylideneamino)benzoic Acid (3e)The product was obtained as yellow powder, mp: 242–244°C; yield %: 54(A); 60 (B). IR (KBr, cm−1): 3415 (OH), 1741, 1678 (C=O), 1602 (C=N); 1H-NMR (CDCl3) δ: 5.00 (2H, s, C6H5CH2), 6.55 (1H, d, J=2.19 Hz), 6.69 (1H, dd, J=8.43, 2.19 Hz), 7.09 (2H, d, J=8.43 Hz,), 7.22–7.36 (6H, m, Ar), 8.23 (2H, d, J=8.43 Hz); 13C-NMR (CDCl3) δ: 42.99, 116.1, 126.1, 127.8, 130.8, 139.4, 153.9, 161.6 (CO), 169.8 (C=N), 178.8 (COOH). Anal. Calcd for C22H15ClN2O3 (390.08): C, 67.61; H, 3.87; N, 7.17. Found: C, 67.87; H, 4.06; N, 7.33.
4-(1-Benzyl-5-fluoro-2-oxoindolin-3-ylideneamino)benzoic Acid (3f)The product was obtained as yellow powder, mp: 216–218°C; yield %: 80 (A); 89 (B). IR (KBr, cm−1): 3415 (OH), 1744, 1678 (C=O), 1603 (C=N); 1H-NMR (CDCl3) δ: 5.01 (2H, s, C6H5CH2), 6.29 (1H, dd, J=8.07, 4.0 Hz), 6.70 (1H, dd, J=8.79, 4.0 Hz), 6.98 (1H, td, J=8.79 Hz), 7.09 (2H, d, J=8.43 Hz), 7.29–7.37 (5H, m, Ar), 8.23 (2H, d, J=8.43 Hz);13C-NMR (CDCl3) δ: 41.7, 115.9, 125.9, 126.69, 127.6, 130.8, 142.1. Anal. Calcd for C22H15FN2O3 (374.37): C, 70.58; H, 4.04; N, 7.48. Found: C, 70.84; H, 4.33; N, 7.77.
Animal BiologyThe adult zebrafish were raised and maintained following the guidelines described in the zebrafish book.44)
Animal TreatmentThe wild type (AB Tubingen) and transgenic Tg(Fli-1: EGFP) zebrafish embryos were obtained by natural pairwise mating and treated as described previously.45) Three biological replicate trials were conducted for each experiment.
SU-5416 (1,3-dihydro-3-[(3,5-dimethyl-1H-pyrrol-2-yl)methylene]-2H-indol-2-one) was purchased from Sigma-Aldrich, Cat # S8442 Sigma lot # 052M4611V.
O-Dianisidine Staining for Erythroid CellsThe presence of erythroid cells were evaluated by O-dianisidine staining, as previously described.46)
Cell Culture and Cell Viability AssayN-Alkyl-isatin-3-iminobenzoic acid derivatives were screened in HepG2, MCF7, and Jurkat cell lines to assess the in vitro cytotoxicity activity by MTT assay as previously described.6)
StatisticsOrigin (Version 6.1052; Origin Lab Corp Northampton, MA 01060, U.S.A.) was used for statistical analysis. The data were considered statistically significant for values of p<0.05.
The authors extend their sincere appreciation to Vice Deanship of Research Chairs, King Saud University, for supporting this study.
The authors declare no conflict of interest.