2018 Volume 41 Issue 3 Pages 360-367
2018 Volume 41 Issue 3 Pages 360-367
Acquired resistance is a major reason for poor clinical outcomes in cancer chemotherapy patients. The aim of this study was to determine the sensitivity to anticancer drugs and to identify the alterations of DNA repair and drug transporter in a model of primary culture obtained from pre- and post-platinum-based anticancer treatments in nine Thai gastric cancer patients. Ex vivo sensitivity to anti-cancer drugs (cisplatin, oxaliplatin, 5-fluorouracil (5-FU) and irinotecan) was analysed using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The expression of the drug transporter (multidrug resistance-associated protein 1 (MRP1), P-glycoprotein (P-gp)) and DNA repair (X-ray cross-complementing gene 1 (XRCC1) and excision repair cross-complementing 1 (ERCC1)) were examined by RT-PCR. The IC50 to cisplatin and oxaliplatin of the cells obtained from gastric cancer patients after clinical drug treatments were administered to five patients (55.5%) revealed a significant increase when compared with prior treatments. The basal expression values of XRCC1, ERCC1 and MRP1 obtained from the treated patients were in correlation with those of IC50. Ex vivo platinum drug treatment of the primary culture obtained from naïve patients over seven days also revealed a significant increase in MRP1 (7/9), XRCC1 (4/9) and ERCC1 (4/9). These observations have also been observed in the KATOIII cell line. Clinical treatment by platinum-based anti-cancer drug can develop acquired drug resistance in Thai gastric cancer patients through upregulation in the expression of drug transporter MRP1 and DNA repair XRCC1 and ERCC1. In cell culture model, cisplatin-resistant gastric cancer cell line KATOIII/diamminedichloroplatinum (KATOIII/DDP) significantly increased the expression level of these genes when compared to its parental cells (KATOIII).
Gastric cancer is the second leading cause of cancer-related deaths worldwide.1) The prognosis for gastric cancer remains poor with a high rate of relapse.2,3) Presently, no standard form of treatment has been approved for gastric cancer.4) The most common treatment used to treat advanced gastric cancer patients consists of fluoropyrimidine coupled with platinum-based chemotherapy.5) However, many patients are intrinsically resistant or develop acquired resistance to the treatment and this often leads to relapses, which have resulted in a shortening of their overall lifespan.6)
For decades, cancer cell lines have been used as a model for investigations into the mechanisms of the standard treatment approaches and the efficacy of anticancer drugs.7,8) However, each patient maintains a different genetic background. In fact, cancer cell lines poorly represent the diversity, heterogeneity, and drug resistant tumors that occur in many cancer patients. Therefore, using primary cultures established from patients’ tumors to investigate the sensitivity of drugs and to search for the predictive markers for drug responses in pre-clinical studies could be translated to clinical trials and beyond. This strategy will provide a more effective benchmark for bedside translation.9)
Among the various chemoresistance mechanisms, the overexpression of drug-resistance proteins including drug transporters and DNA repairing systems are considered an important defense mechanism of cancer cells in their resistance against chemotherapeutic drugs. Drug efflux transporters of the ATP-binding cassette transporter family such as the ABCB1 (multidrug resistance 1, MDR1, P-glycoprotein (P-gp)) and the ABCC (multidrug resistance-associated protein (MRP)) family are capable of regulating the intracellular concentration and tissue distribution of drugs and their metabolites which are known to lead to drug resistance.10) Platinum drugs cause DNA damage by forming intrastrand and interstrand platinum-DNA cross-links.11) Increased levels of DNA repair inhibit the effects of cisplatin-induced DNA damage and cause chemotherapy resistance. Many recent studies have shown that molecules involved in DNA repair pathways, such as the excision repair cross-complementing 1 (ERCC1) and X-ray cross-complementing gene 1 (XRCC1), are related to poor clinical outcomes in many forms of cancer such as head and neck, colorectal and ovarian cancer, as well as gastric cancer.12–15) However, there is only a limited amount of data available that is concerned with changes in the expression profile of the drug transporter and the repair of DNA genes in gastric cancer patients after chemotherapeutic treatments. In our study, the primary cultures from nine pairs of endoscopic biopsies obtained from Thai gastric cancer patients before and after clinical chemotherapy treatments by Folinic acid+Fluorouracil and Oxaliplatin (FOLFOXIV) (oxaliplatin+5-fluorouracil (5-FU)/leucovorin) were examined. The ex vivo chemosensitivity to anticancer drugs (cisplatin, oxaliplatin, 5-FU and irinotecan) was examined. The alteration change on mRNA expression of the drug transporter (MRP-1, P-gp), and the DNA repair (XRCC1, ERCC1) in nine pairs of the primary culture at the basal levels and at the ex vivo treatment stage were investigated using RT-PCR. The correlation between the interval changes on mRNA expression at the basal levels and the ex vivo drug treatment was investigated. The correlation between the expression of the drug transporter and the DNA repair and chemosensitivity to anticancer drugs was also investigated. It is predicted that the quantification of gene expression levels may be helpful in guiding therapy decisions and anticipating individual tumor responses towards chemotherapeutic treatments.
Dulbecco’s modified Eagle’s medium (DMEM), penicillin–streptomycin, and trypsin–ethylenediaminetetraacetic acid (EDTA) were purchased from GIBCO-BRL (Grand Island, NY, U.S.A.). Fetal bovine serum (FBS) was purchased from Hyclone (Logan, UT, U.S.A.). Cisplatin, oxaliplatin and 5-FU were purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.). Cisplatin stock solutions were prepared at concentrations of 5 mM. 5-FU and oxaliplatin stock solutions were prepared at a concentration of 10 mM. For each experiment, all drugs were freshly prepared and diluted with the appropriate solvent to achieve the final treatment concentrations.
Statement of EthicsAll studies were approved by the Ethics Committee for Human Research, Faculty of Medicine, Chiang Mai University (Ethics number 408/2015), Thailand. Written informed consent by the patients was obtained prior to use of the endoscopic biopsy samples.
PatientsEndoscopic biopsies of nine pairs of tissue specimens both before and after chemotherapeutic treatment with FOLFOXIV (Oxaliplatin+5-FU/leucovorin) for gastric cancer patients were obtained from the Department of Surgery, Maharaj Nakorn Chiang Mai Hospital, Chiang Mai, Thailand along with written informed consent of all participants. All specimens were processed within a day of the sample collection process. The clinical features of all patients, including age at diagnosis, gender and histological type were obtained from clinical and pathological reports. All patients were diagnosed pathologically according to the criteria of WHO.
Primary Gastric Culture from Endoscopic BiopsyThe endoscopic biopsy tissue samples (size ca. 2 mm3) were added into the enzyme mixture (Collagenase A 0.15 U/mL, Dispase II 2.4 U/mL and bovine serum albumin (BSA) diluted in incomplete medium) and mixed every 10 min for 60 min.16) After that, the tissue was then pelleted by centrifugation at 4500×g for 5 min and the supernatant was discarded. The cells were washed two times with 10 mL of DMEM. The cell suspension mixture was added into a matrix-coated six-well-plate containing 10% FBS and DMEM. The medium was changed 24 h after the initial plating and then every three days.
Establishment of Cisplatin-Resistant KATOIII Cell LinesIn order to compare sensitive and resistant phenotypes within the context of the same genetic background, we derived sublines from parental KATOIII cell lines that were known to be resistant to cisplatin. KATOIII cells were established to acquire resistant cells (KATOIII/diamminedichloroplatinum (KATOIII/DDP)) via a step-wise increase in the cisplatin concentration from 0.5 to 3 µM over 10 months.17) The resistance ratio was then determined. Before each experiment, the KATOIII/DDP cells were cultured in drug-free DMEM medium for two passages.
Chemosensitivity TestPrimary gastric culture (2.5×103 cells), KATOIII and KATOIII/DDP cell lines (5×103 cells), were plated in a 96-well plate. After 24 h, various concentrations (0–100 µM) of anticancer drugs (cisplatin, oxaliplatin, 5-FU and irinotecan) were added. KATOIII cells and KATOIII/DDP cells were incubated for 72 h. With regard to the primary culture, the culture medium was changed after 48 h and incubated until Day 5. The culture supernatant was removed and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) dye was added to the mixture and it was incubated for an additional 4 h. MTT-formazan was dissolved in dimethyl sulfoxide (DMSO) and absorbance was measured using a microplate reader at 540 nm with a reference wavelength of 630 nm.18) Optical density was found to be directly proportional to the number of living cells present in the culture. The IC50 value was defined as the concentration of the drug required to kill 50% of the cells.
RNA Extraction and Real-Time PCRTotal RNA was extracted using Isogen (Molecular Research Center Inc., U.S.A.). RNA was reverse transcribed to cDNA using a ReverTra Ace® qPCR RT Kit (Toyobo, Japan). Real-time PCR was carried out on Eppendorf equipment using SYBR Green® Realtime PCR Master Mix (Toyobo) was employed in accordance with the manufacturer’s protocols. The results were analyzed by the 2-ΔCt method.19) The primer sequences were as follows: 5′-ACT GCT GGA ACC TGG CCC TGC-3′ (forward) and 5′-GCA AAC CCC GAG GAG AAG GCA-3′ (reverse) for XRCC1; 5′-AAG TGC TGC GAG CCC TGG GC-3′ (forward) and 5′-AAT AAG GGC TTG GCC ACT CC-3′ (reverse) for XRCC1, for ERCC1; 5′-CGG AAA CCA TCC ACG ACC CTA ATC-3′ (forward) and 5′-ACC TCC TCA TTC GCA TCC ACC TGG-3′ (reverse) for MRP1; 5′-CTT GGC AGC AAT TAG AAC-3′ (forward) and 5′-TCA GCA GGA AAG CAG CAC-3′ (reverse) for P-gp; 5′-CCC CTT CAT TGA CCT CAA CTA C-3′ (forward) and 5′-GAT GAC AAG CTT CCC GTT CTC-3′ (reverse) for glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Statistical AnalysisAll statistical analyses were performed using SPSS22.0 software (Chicago, IL, U.S.A.). The experiments were performed in triplicate. Quantifications are defined as mean±standard deviation (S.D.) of three independent experiments. The Student’s t-test was used to test whether or not the differences identified between the two groups were significant. The correlation between XRCC1, ERCC1, MRP1, P-gp and chemosensitivity was analyzed by Pearson’s correlation coefficient. Two-tailed, differences were considered significant when * p<0.05 and ** p<0.01.
Characteristics of the nine gastric cancer samples are shown in Table 1. The patients had undergone endoscopic biopsies at the Maharaj Nakorn Chiangmai Hospital (Chiang Mai, Thailand) between June 2014 and June 2016. The median age was 54.3 years (ranging from 41 to 77 years). The ratio of males to females was 6 : 3. The tumor tissue samples were immediately collected following endoscopic biopsies. Each of the diagnoses was histopathologically confirmed by a senior pathologist. Patients No. 1, 2, 4 and 8 were moderately differentiated and patients No. 3, 5, 6, 7 and 9 were poorly differentiated. Figure 1 presents pictures of a tumor biopsy and the primary culture obtained from patients.
| Patient No. | Gender | Age | Histological type | Compound | IC50 | |
|---|---|---|---|---|---|---|
| Non-treated | Clinical treatment | |||||
| 1 | Male | 54 | Moderate | Cisplatin | 5.00±0.71 | 13.50±0.71* |
| Oxaliplatin | 6.00±0.82 | 10.00±1.06* | ||||
| 5-FU | 10.50±0.35 | 10.00±0.00 | ||||
| Irinotecan | 7±0.23 | 6±0.57 | ||||
| 2 | Female | 71 | Moderate | Cisplatin | 12.50±0.35 | 25±0.00* |
| Oxaliplatin | 8.50±1.80 | 25±0.00* | ||||
| 5-FU | 25.00±2.82 | 25±0.26 | ||||
| Irinotecan | 8±0.57 | 17±0.00* | ||||
| 3 | Male | 50 | Poor | Cisplatin | 11.00±0.97 | 18.50±0.00* |
| Oxaliplatin | 8.25±1.30 | 25.00±1.25* | ||||
| 5-FU | 6±0.23 | 25.00±1.20* | ||||
| Irinotecan | 18±0.23 | 15±0.00 | ||||
| 4 | Male | 50 | Moderate | Cisplatin | 21±3.5 | 10.5±1.5* |
| Oxaliplatin | 21±1.12 | 15±1.56* | ||||
| 5-FU | 25±2.43 | 12.5±2.23* | ||||
| Irinotecan | 25±0.00 | 25±0.78 | ||||
| 5 | Female | 44 | Poor | Cisplatin | 15±1.34 | 18±0.67 |
| Oxaliplatin | 7±2.56 | 14.5±0.23* | ||||
| 5-FU | 3±0.34 | 6.5±1.54* | ||||
| Irinotecan | 6±0.53 | 10±0.53* | ||||
| 6 | Male | 77 | Poor | Cisplatin | 17±0.57 | 15±1.34 |
| Oxaliplatin | 10±0.00 | 23±1.65* | ||||
| 5-FU | 9±0.00 | 25±0.00* | ||||
| Irinotecan | 6±0.48 | 8±1.23 | ||||
| 7 | Female | 45 | Poor | Cisplatin | 8±0.98 | 10±0.12 |
| Oxaliplatin | 25±0.00 | 25±0.00 | ||||
| 5-FU | 25±0.00 | 25±0.00 | ||||
| Irinotecan | 6±0.17 | 6±0.00 | ||||
| 8 | Male | 57 | Moderate | Cisplatin | 7±0.34 | 10±0.67* |
| Oxaliplatin | 5±0.00 | 5±0.00 | ||||
| 5-FU | 5±0.67 | 7.5±0.23 | ||||
| Irinotecan | 9±0.00 | 7±0.53 | ||||
| 9 | Male | 41 | Poor | Cisplatin | 11±0.65 | 15±0.79* |
| Oxaliplatin | 8±0.67 | 10±0.87 | ||||
| 5-FU | 6±0.98 | 6±0.11 | ||||
| Irinotecan | 4±0.00 | 5±0.53 | ||||
Compared with non-treated group, * p<0.05.

The morphology of the primary gastric culture was first examined using a light phase-contrast microscope (10×). Picture of endoscopic biopsy sample obtained from patients (A). Primary gastric culture on Day 2 after plating (B). Eighty percent confluence of primary gastric culture on Day 7 (C).
In this study, we assessed the sensitivity of nine pairs of primary culture obtained from endoscopic biopsies before and after clinical chemotherapeutic treatment to different chemotherapeutic drugs including cisplatin, oxaliplatin, 5-FU and irinotecan. Experimentation was done involving the primary culture ex vivo using MTT assay over five days. Measurement of IC50 was performed to evaluate the sensitivity of the primary culture to chemotherapeutic drugs. The results are shown in Table 1. Cisplatin was found to be cross-resistant to oxaliplatin. After patients had received the drug for 6 cycles, IC50 values of cisplatin and oxaliplatin from patients No. 1, 2, 3, 5 and 9 were significantly increased when compared to prior treatments (p<0.05). Patient No. 4 showed significantly decreased IC50 values for the platinum drugs. IC50 values for 5-FU from patient No. 4 also significantly decreased while patients No. 3 and 5 showed increased IC50 values for 5-FU. Another drug of choice for the treatment of gastric cancer patients is irinotecan. Patients No. 2 and 5 showed significantly increased IC50 values when compared to prior treatments. These results suggest that some patients displayed a trend of greater resistance to standard drugs after receiving clinical drug treatments. Notably, patient No. 5 displayed increased IC50 values for all four drugs.
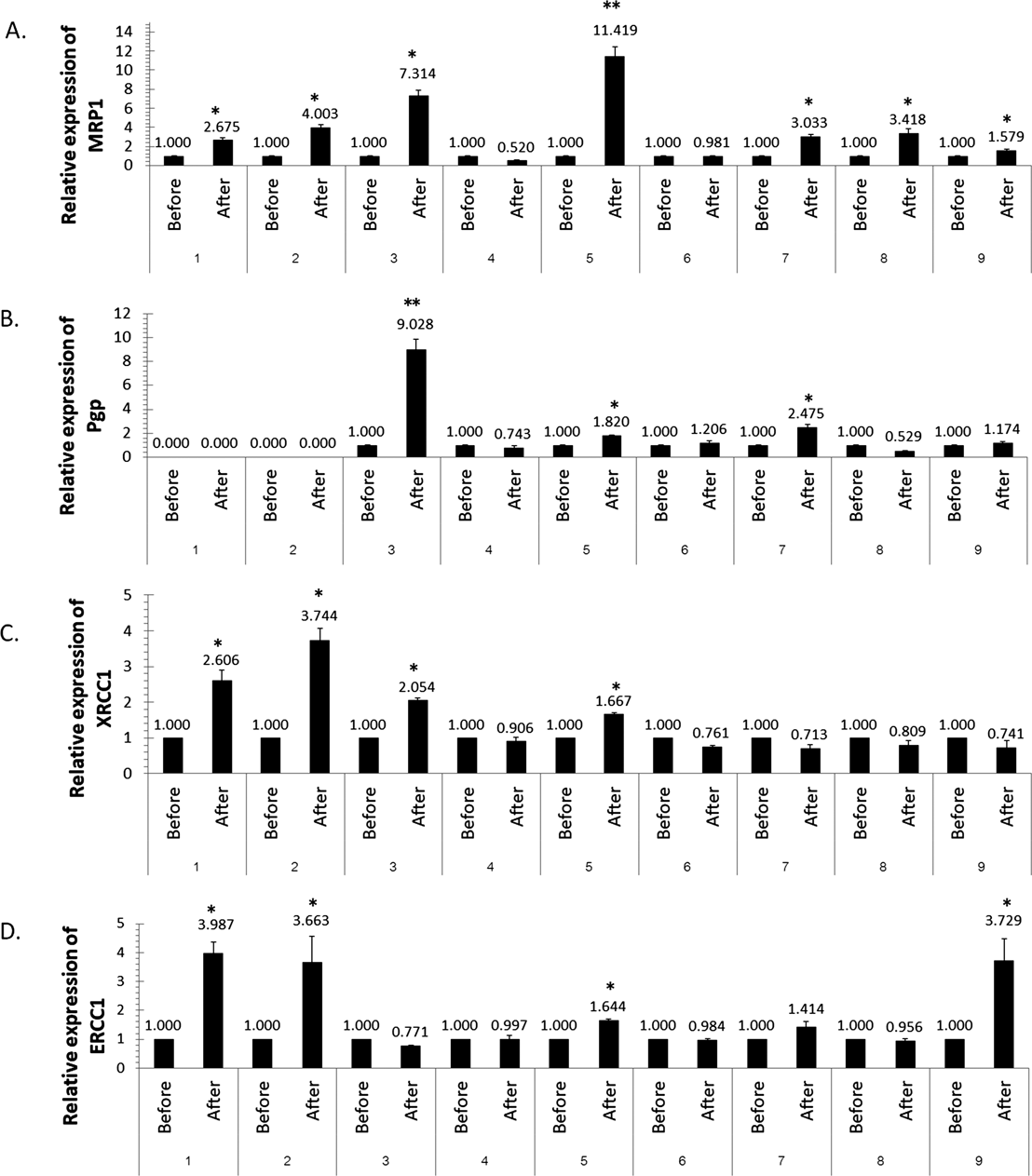
The Expression of Drug Transporter (MRP1, P-gp) and DNA Repair (XRCC1, ERCC1) of Nine Pairs Primary CulturesThe next step of our study involved analyzing the alteration changes in the mRNA expression of drug transporter (MRP1 and P-gp) and DNA repair (XRCC1 and ERCC1) in the primary culture due to the clinical chemotherapy treatment with FOLFOXIV (oxaliplatin+5-FU/leucovorin) over six cycles. The results of mRNA expression are shown in Fig. 2. The clinical chemotherapeutic treatment significantly elevated mRNA expressions of MRP1 (7/9), P-gp (4/9), XRCC1 (4/9), ERCC1 (4/9) when compared with prior treatments (p<0.05). When comparing the sensitivities of the primary culture of drugs with the alteration on gene expression, there were directly significant correlations between the IC50 value of the platinum drugs (cisplatin and oxaliplatin) and the alterations of XRCC1 and ERCC1 expressions in patients No. 1, 2, 3, 4, 5 and 9 (p<0.05), while no statistical differences were found with the IC50 values of 5-FU and irinotecan (p>0.05).

The mRNA levels of MRP1 (A), P-gp (B), XRCC1 (C), and ERCC1 (D) were measured by RT-PCR. The relative level of each target gene mRNA was determined after normalization to GAPDH mRNA. The mRNA levels of control group without clinical drug treatment were set to 1.00. Values are expressed as the mean±S.D. * p<0.05, ** p<0.01 significantly different compared with the value for the non-treated group.
For ex vivo and in vivo comparisons at the individual patient level, not all drugs received by the patients were tested due to a limited amount of primary culture cells. Cisplatin was used in this experiment as a surrogate of the first-line drug. Non-toxic concentrations of cisplatin at 3 µM were used in this experiment and incubated with the primary culture obtained from naïve patients for seven days. The expression of the drug transporter and DNA repair was detected by RT-PCR. The results are shown in Fig. 3. The ex vivo cisplatin treatment significantly elevated the mRNA expression of MRP1 as was detected in seven of nine patients, XRCC1 in four of nine patients, ERCC1 in four of nine patients and P-gp in three of nine patients. Ex vivo cisplatin treatment on the alteration on the expression of MRP1, XRCC1, ERCC1 and P-gp showed a correlation with the relevant data obtained from the clinical chemotherapy treatment (p<0.05).
Primary gastric cultures from naïve patients were treated with 3 µM of cisplatin for 7 d. The mRNA levels of MRP1 (A), P-gp (B), XRCC1 (C), and ERCC1 (D) were measured by RT-PCR. The relative level of each target gene mRNA was determined after normalization to GAPDH mRNA. The mRNA levels of control group without ex vivo cisplatin treatment were set to 1.00. Values are expressed as the mean±S.D. * p<0.05, ** p<0.01 significantly different compared with the value for the non-treated group.
Primary gastric cells obtained from patients can be used for not more than seven passages in the experimental process. Therefore, in order to investigate the underlying mechanisms and to compare the sensitive and resistant phenotypes in the same genetic background, we have derived sublines from the human gastric cancer cell line KATOIII that are resistant to cisplatin. After the KATOIII cells have been continuously grown in the presence of step-wise increased concentrations of cisplatin, the chemosensitivity of the sensitive and cisplatin resistance sublines were determined by MTT assay. The IC50 values of cisplatin were found to be 4 and 37 µM for KATOIII and KATOIII/DDP, respectively. Under our experimental conditions, the KATOIII/DDP cell line was approximately 9.25 more resistant to cisplatin. We also detected the sensitivity of these cells to oxaliplatin, 5-FU and irinotecan. IC50 of oxaliplatin were found to be 4 and 13 µM for KATOIII and KATOIII/DDP, respectively (Resistance ratio: 3.25). The IC50 of irinotecan was found to be 25 and 50 µM (resistance ratio: 2). Both cell lines revealed the same level of drug sensitivity to 5-FU (Fig. 4).

KATOIII cells and KATOIII/DDP cells were incubated with four standard drugs at 0–100 µM for 72 h. The chemosensitivity of by MTT assay on day 3 are presented; cisplatin (A) oxaliplatin (B) 5-FU (C) and irinotecan (D). The expression of mRNA expression of drug transporter MRP1(E) and DNA repair XRCC1 (F), ERCC1(G) using RT-PCR is shown. Values are expressed as the mean±S.D. * p<0.05 significantly different compared with the value for KATOIII cell.
To investigate the expression of XRCC1, ERCC1, P-gp and MRP1 in sensitive and resistant-cells, RT-PCR was performed. The results revealed that the expression of XRCC1, ERCC1 and MRP1 of the resistant KATOIII cell lines increased by 2.55, 1.765 and 18.152 fold when compared to the sensitive KATOIII cell lines. P-gp was not detected in both the KATOIII and KATOIII/DDP cells.
Chemoresistance is a major problem that has been known to lead to treatment failure and death in gastric cancer patients. Among the various chemoresistance mechanisms, overexpression of drug-resistance proteins including drug transporter and DNA repairing systems are important defence mechanisms of cancer cells against chemotherapeutic drugs. Drug efflux transporters including the ABCB1 (P-gp, MDR1) and the ABCC1 (MRP1) family are capable to reducing the intracellular drug concentration. Several reports have indicated the resistance to 5-FU and irinotecan due to the MRP associated cellular efflux processes.20,21)
With regard to the DNA repairing system, this study focused on XRCC1 and ERCC1 based on the approaches of previous studies. X-Ray repair cross-complementing 1 (XRCC1) is required for repair of DNA single-strand breaks (SSBs) to eliminate the DNA damage induced by chemical mutagens and ionizing radiation. The repair cross-complementation group 1 (ERCC1) plays a critical role in excision repair. Several studies have shown that the XRCC1 and ERCC1 genes are correlated with the sensitivity of drug in many cancer type and clinical efficacy. XRCC1 negative cells were found to be sensitive to cisplatin when compared to XRCC1 positive cells and the reduced expression of XRCC1 in gastric cancer tissues, which correlates with a significant survival benefit associated with adjuvant first-line platinum-based chemotherapy.22)
There is limited available data concerning changes in the expression of drug resistance-related gene in gastric cancer patients after chemotherapeutic treatment. To investigate the role of chemotherapeutic agents on gene expression, we quantified the mRNA expression after chemotherapy treatment. The expression analysis of the basal levels of drug transporters (MRP1, P-gp) and DNA repair (XRCC1, ERCC1) was performed using RT-PCR in the primary culture obtained from patients before and after undergoing chemotherapeutic treatment. All nine primary cultures showed high expressions of MRP1, XRCC1 and ERCC1, while P-gp displayed no expression in two patient samples at the lowest level of detection even after undergoing chemotherapeutic treatment. Taxane drugs including docetaxol and paclitaxel have been known as a substrate of MRP1 and P-gp. Based on our finding, we suggested that it should be concerned to use taxane drugs as second-line treatment after use platinum and 5-FU as a first-line drug. Moreover, the basal expression of MRP1 and P-gp have been detected in about 56.09% (23/41) and 29.2% (12/41) in gastric cancer samples (supplementary Fig. S1). Therefore, treatment the gastric cancer patient with taxane chemotherapeutic drugs should be concerned the level of MRP1 and P-gp. Although high expression of MRP1 and P-gp have been found in the samples, the anticancer drugs that are usually used in the treatment of gastric cancer in Thailand were not the targets for this type of drug transporter.
There are several kinds of anti-cancer drug regimens for gastric cancer treatment world-wide such as platinum-drug cisplatin and oxaliplatin, irinotecan, doxetaxol, paclitaxel, and trastuzumab. However, in this study we focused the effect of chemotherapeutic drugs that have been used in Thai gastric cancer patients. From several reports indicated that combination treatment of trastuzumab and capecitabine or 5-FU and cisplatin has been shown to be beneficial to HER-2-positve gastric cancer patients. However, the percent of HER-2 overexpression in Thai gastric cancer patients demonstrated only 9% (20 in 224).23) Our first-line drug treatment is a platinum-based drug in combination with 5-FU and the second-line drug is irinotecan. Therefore, we have selected four drugs including cisplatin, oxaliplatin, 5-FU and irinotecan in this study to generate the understanding of the genetic expression and alteration after clinical chemotherapeutic treatment which could improve gastric cancer treatment in Thai gastric cancer patients.
Sensitivity to four standard drugs including cisplatin, oxaliplatin, 5-FU and irinotecan were detected in the primary culture obtained from gastric cancer patients before and after undergoing chemotherapy treatment. The results showed that after patients had received the drug, the cells displayed a greater level of resistance to chemotherapy when compared to the prior treatment cases. Moreover, the basal level of the primary culture after treatment and the ex vivo chemotherapy treatment showed increases in the expressions of MRP1 (7/9), P-gp (3/9) XRCC1 (4/9) and ERCC1 (4/9). These expressions correlated with the MTT results. The data indicates that enhanced drug transporter and DNA repair capacity could be the major mechanisms for developing drug resistance in gastric cancer patients. Similar results with regard to the cisplatin-resistant KATOIII cell line demonstrated the most pronounced expression of MRP1 (18.15 fold), followed by XRCC1 (2.55 fold) and ERCC1 (1.77 fold) when compared to the cisplatin-sensitive KATOIII cell line. These results suggest that the expressions of XRCC1, ERCC1 and MRP1 are in accordance with the acquired drug resistance and could be markers for chemotherapy resistance in gastric cancer patients. Although the upregulation on the expression of DNA repair XRCC1,24) ERCC1,25) and drug transporter MRP126) have been reported in cisplatin-resistance gastric cancer cell lines. Here is the first report in real clinical sample from patients. However, a greater sample size is needed to produce results that would be clinically relevant to treatment and to confirm predictions on chemotherapy resistance or response as a new biomarker for advanced gastric cancer treatment. Moreover, the mechanistic study in more details including signaling pathways that might involve in drug transporter and DNA repair between KATOIII and KATOIII/DDP cell line will be further investigated.
In conclusion, our results showed that MRP1, XRCC1 and ERCC1 seem to be involved in the drug resistance that occurs among Thai gastric cancer patients with the most pronounced effect being on MRP1, followed by XRCC1 and ERCC1. Moreover, cisplatin-resistant gastric cancer cell line (KATOIII/DDP) also showed significantly increase the level of these genes when compared to its parental cells (KATOIII). Thus, the quantification of the drug transporter and DNA repair expression before and after chemotherapeutic treatment might be an important step for improving the effectiveness of chemotherapy and help guide the doctor in selecting the right chemotherapy for more individualized treatment and the prediction of individual tumor responses. Moreover, the development of the specific inhibitors of MRP1, XRCC1 and ERCC1 might increase chemotherapeutic effectiveness.
This work has been supported with funding provided by the 50th Anniversary of Chiang Mai University Research Fund (Ph.D. 003/2556), Center for Research and Development of Natural Products for Health and the National Research Council of Thailand (NRCT, 2560A10402045). We would like to thank Dr. Russel Kirk Hollis from Faculty of Humanities, Chiangmai University for checking the English grammar in our manuscript.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials. Figure S1. Drug transporter P-gp and MRP1 mRNA expression in 41 Thai gastric cancer patients using real-time PCR. (A) Graph showing the levels of P-gp mRNA in the primary gastric culture, (K562/ADR; positive control). (B) Graph showing the levels of MRP1 mRNA levels on primary gastric culture (KATOIII; positive control).