2018 Volume 41 Issue 5 Pages 690-696
2018 Volume 41 Issue 5 Pages 690-696
The purpose of this study was to determine the effects of different concentrations of ligustrazine, an extract from Chinese herb, on ketamine requirement for hypnosis and analgesia in mice. In the hypnotic response study, mice were randomly allocated to receive saline or ligustrazine at 10, 20, 40, 80 or 160 mg·kg−1 by intraperitoneal injection. Ketamine was administrated 15 min after ligustrazine injection. The hypnotic response was determined by assessing loss of the righting reflex (LORR) after ketamine injection. The dose of ketamine was determined by modified Dixon’s up-and-down method in each group. In the analgesia study, different doses of ligustrazine were administrated 15 min before 50 mg·kg−1 ketamine injection. The analgesia effects (pain threshold) were determined by heat radiation-induced tail-flick latency and evaluated before ligustrazine administration or 5, 15, 30 and 60 min after ketamine administration. The ED50 [95% confidence interval (CI)] for hypnosis induced by ketamine was 54.1 (44.8, 65.3) mg·kg−1. Ligustrazine dose-dependently decreased the ED50 for ketamine to induce hypnosis, which was [31.6 (26.2, 38.1)] mg·kg−1 with the addition of 80 mg·kg−1 ligustrazine and [27.7 (22.6, 33.7)] mg·kg−1 with the addition of 160 mg·kg−1 ligustrazine, respectively (p<0.05). Ligustrazine at 160 mg·kg−1 also increased pain threshold in the presence of ketamine. Ligustrazine enhanced the hypnotic effect of ketamine in a dose-dependent manner. Ligustrazine at a large dose also increased the analgesic effect of ketamine.
Ketamine is an intravenous anesthetic that acts primarily via antagonizing glutamatergic N-methyl-D-aspartate (NMDA) receptors.1,2) It is a sedative-hypnotic agent with powerful analgesic properties and produces unconsciousness in 30 to 60 s. The effects are terminated in 15 to 20 min after an intravenous induction dose. Because of short-term effect and alleviating bronchospasm by a sympathomimetic effect, ketamine is widely used in pediatric or asthmatic patients for surgery.3) There is a renewed interest as ketamine can function as an analgesic by blocking the NMDA receptors involved in nociceptive and inflammatory pain transmission.4–6) In emergency departments, ketamine is frequently used for procedural sedation and intubation.7,8) Moreover, ketamine is considered as a first-line treatment for severely agitated emergency department patients.9,10) Ketamine anesthesia is also applied for electroconvulsive therapy in patients with depression.11)
The NMDA receptor is a kind of central nervous system excitatory amino acid ionotropic receptors.12,13) Activation of the NMDA receptor requires binding two kinds of amino acid including glutamate and its co-agonist of glycine.14,15) Once NMDA receptor in the postsynaptic membrane is activated, it promotes transmembrane Ca2+ influx through L-type voltage-gated calcium channels which resulting in intracellular Ca2+ ([Ca2+]i) overload.16,17) As a noncompetitive antagonist of the NMDA receptor, ketamine can effectively reduce the [Ca2+]i by suppressing Ca2+ influx.17)
Ligustrazine whose chemical structure is tetramethylpyrazine (TMP) is a bioactive ingredient extracted from the Chinese herb Chuanxiong (Rhizoma Chuanxiong),18) which has been extensively used for medicinal purpose for more than 2000 years. As one of the popular Chinese Patent Medicine (CPM), ligustrazine has been widely used as an adjunctive therapy of cardiovascular diseases in China.19,20) Moreover, ligustrazine demonstrated a definite clinical efficacy in improving neurological outcome after brain ischemia in patients without obvious adverse events.21) In the past decades, researchers explored other pharmacological capabilities of TMP in various diseases, such as diabetes,22) cancers,23) and liver injury.24) As a result, laboratory study has shown the regulation ability of this agent at multiple molecular targets, such as anti-inflammation, antioxidant, antiplatelet, and antiapoptosis.25) Some evidence have indicated that the protective mechanism of ligustrazine is related to inhibit the release of excitatory amino acids and attenuate [Ca2+]i overload by supppressing Ca2+ influx.26,27)
It is a common phenomenon in China that patients with cardiovascular or cerebral diseases may have received ligustrazine treatment before anesthesia and surgery. Since both ketamine and ligustrazine reduce Ca2+ influx, whether ligustrazine affects the potency of anesthetics is still undetermined. Our present study is to explore whether and how ligustrazine influences the hypnotic and analgesic effects of ketamine in mice.
This study was approved by the animal care committee at Sun Yat-sen University and performed in accordance with the National Institutes of Health Guide for the Use of Laboratory Animals. Four-month old C57BL/6 male mice (Guangdong Medical Laboratory Animal Co., China, permission number: SCXK20130002) weighing 22–25 g, were housed in cages and kept under temperature-controlled environmental conditions on a 12 : 12 constant light–dark cycle. They also had free access to food and water. One hundred and fifty-five mice in total were enrolled in this study and they were randomly allocated into experimental groups.
Experimental ProtocolLigustrazine and ketamine hydrochloride were dissolved freshly in 0.9% saline solution on the day of the experiment. They were administered intraperitoneally in a volume of 0.1 mL per 10 g of body weight. The effects of ligustrazine on the anesthetic potency of ketamine were evaluated using two endpoints: loss of righting reflex (LORR, as a measure of hypnosis) and tail-flick latency induced by heat radiation (as a measure of analgesia effect). In experiment one, five doses of ligustrazine (10, 20, 40, 80, or 160 mg·kg−1) or saline were administered 15 min before ketamine (n=12–13 in each group). Ketamine-induced hypnosis was determined by LORR via up-and-down sequential method to define the ED50. The effects of different doses of ligustrazine on the ED50 of ketamine-induced hypnosis were determined. Another group of mice were administrated with 160 mg·kg−1 ligustrazine (n=10), LORR were also evaluated for 10 min to test whether ligustrazine alone has hypnotic effect. In experiment two, the effects of ligustrazine on ketamine-induced analgesia were evaluated. Four doses of ligustrazine (20, 40, 80, or 160 mg·kg−1) or saline were administered by intraperitoneal injection 15 min before administration of 50 mg·kg−1 ketamine (n=10). One group received saline for two times as control group (n=10) and one group received saline and 160 mg·kg−1 ligustrazine alone to evaluate the analgesic effects of ligustrazine (n=10).
Losing of Righting Reflex DeterminationLORR was used for assessments of the levels of sedation in this study. As described before,28) the mice were examined individually in a circular glass beaker (13.5 cm diameter×19 cm high) immediately following administration of ketamine or saline. We lay the beaker horizontally on a shelf and rotated it slowly by hand. The righting time was assessed and recorded for 10 min by a blinded observer. LORR was defined as no righting within 10 s. The starting time and the duration of LORR were also measured after administration of drug.
Determining ED50 of Ketamine for Hypnotic EffectThe ED50 of ketamine for its hypnotic effect was measuring by Dixon and Massey up-and-down sequential method in the different groups as described before.29) Dixon’s up-and-down method is a classic pharmacology trial to determine ED50. It can make full use of the information provided by the data and reduce the number of experimental cases by 30–40%, Spacing between adjacent dosage should be set usually on a logarithmic scale. The first dose administrated should be as close as possible to the ED50 value. In our experiment, the dosing modifications for increments or decrements were according to geometric progression (in proportion of 1 to 0.7), nine concentration gradients of ketamine were used: 200, 140, 98, 68.6, 48, 33.6, 23.53, 16.47, and 11.5 mg·kg−1. The first mouse in each group was started with the dose of 48 mg·kg−1 ketamine according to our primary experiments. The dosing administrated to the next mouse was adjusted by reaction of the former mouse. A positive response was defined as LORR after administration of ketamine. If the response of a mouse was positive, the ketamine dose given to the next mouse was decreased to a lower gradient; whereas if the response of a mouse was negative, the ketamine given to the next mouse was increased to a higher gradient. The experiments for each group will be terminated till achieving four positive–negative crossovers.
Tail-Flick TestThe pain threshold was evaluated by recording the latency to tail-flick in reaction to noxious skin heating by a tail-flick apparatus (IITC Life Science Series 8, U.S.A.) as described before.30) The thermal intensity of the tail-flick apparatus was set at degree 35, corresponding to a temperature of 50°C. Furthermore, the cut-off time for maximum latencies was set at 10 s to avoid tail tissue damage. The location of tail-flick thermal stimulus was 3 cm from the tip of the tail. Baseline pain latencies were determined for all groups 15 min before ligustrazine administration and pain latencies were recorded 5, 15, 30, and 60 min after ketamine administration. The tests were repeated for three times with 30 s interval between tests. The average of latencies was taken as final latencies at that time point.
Statistical AnalysisThe sample size for evaluating ED50 of ketamine-induced hypnosis was based on previous literature,31) which has demonstrated that at least four independent pairs of mice with positive/negative LORR should provide reliable estimates of optimal dose of ketamine for hypnotic effect using the up-and-down method of Dixon.31) Therefore, the number of evaluating ED50 of ketamine is not set before the study. The sample size for evaluating latency of tail-flick was calculated to detect a difference of 30% between means with an 80% power at a significance level of 0.05 using the PASS 11 software.
Statistical analyses were performed using the statistical package (SPSS 11.0 for windows). The ED50 of ketamine to induce hypnosis was determined by Dixon and Massey up-and-down methods to calculate the ED50 and 95% confidence interval (CI) of ketamine for LORR. Probit analysis was another method used to calculate the ED50, ED95, and 95% confidence interval for the hypnotic effect and to estimate the curves of the dose–response relations. Tail-flick latency is presented as mean±standard deviation (SD) and analyzed by repeated measures ANOVA with Tukey’s multiple comparisons. Statistical significance was accepted at p<0.05.
Dose–response data were illustrated in Fig. 1. Ligustrazine alone (160 mg·kg−1) could not induce LORR in all rats untill 10 min, therefore no dose–response data showed in the Fig. 1. Based on the Dixon and Massey up-and-down methods, the ED50 of ketamine-induced hypnosis was 54.1 (38.4–76.1) mg·kg−1. From probit analysis (Fig. 2A), the ED50 and ED95 values (95% confidence limits) were 54.1 (44.8–65.3) mg·kg−1 and 74.2 (62.0–100.4) mg·kg−1, respectively. Pretreatment with ligustrazine dose-dependently decreased the ED50 and ED95 values of ketamine-induced hypnosis in mice. The reduction of ED50 and ED95 values was significant in the mice pretreated with 80 or 160 mg·kg−1 ligustrazine, which was almost 50% lower than that of mice administrated with ketamine alone (p<0.05) (Table 1). There was no significant difference in the ED50 and ED95 values between mice pretreated with 80 and 160 mg·kg−1 ligustrazine. The dose–response curve from the probit analysis (Fig. 2B) showed that ligustrazine shifted the curve to the left in a parallel manner, suggesting that ligustrazine enhances ketamine-induced hypnotic effect in a dose-dependent manner.
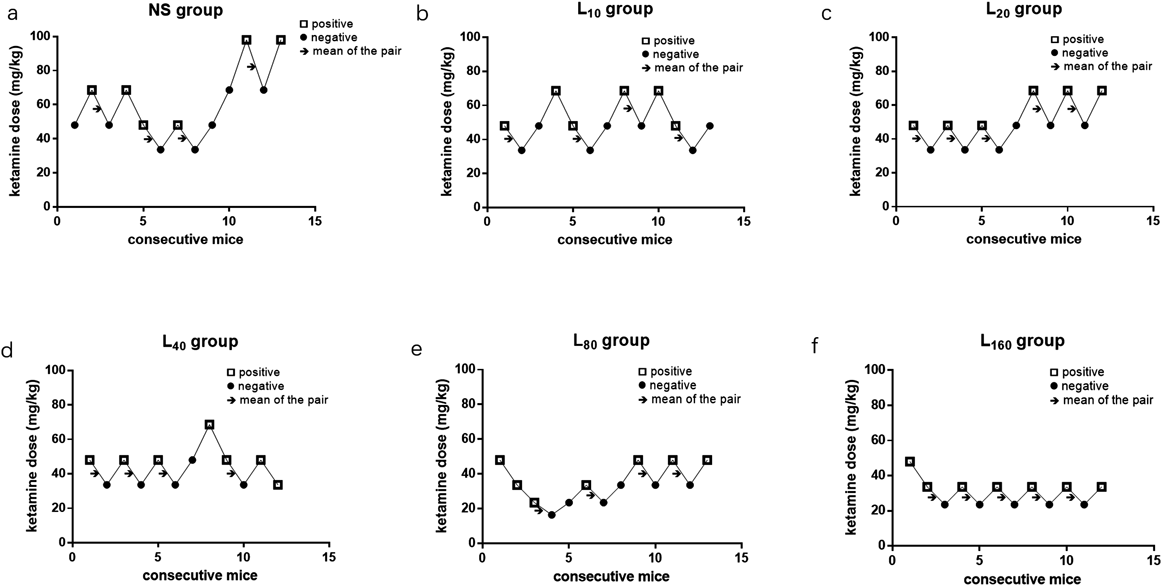
Ligustrazine or normal saline were administered 15 min before ketamine. The first mouse in each group was started with the dose of 48 mg·kg−1 ketamine, the dosing administrated to the next mouse was decreased to a lower gradient if LORR of mice was positive, otherwise an higher gradient dosage of ketamine was administrated. “□” indicated positive response of LORR and “●” indicated negative response of LORR. Arrows indicated the midpoint ketamine concentrations of all independent pairs of mice which manifested crossover from ‘positive’ to ‘negative’ for LORR. The experiments for each group will be terminated till achieving four or five positive–negative crossovers. NS: normal saline; Ket: ketamine; L10: ligustrazine 10 mg·kg−1; L20: ligustrazine 20 mg·kg−1; L40: ligustrazine 40 mg·kg−1; L80: ligustrazine 80 mg·kg−1; L160: ligustrazine 160 mg·kg−1.
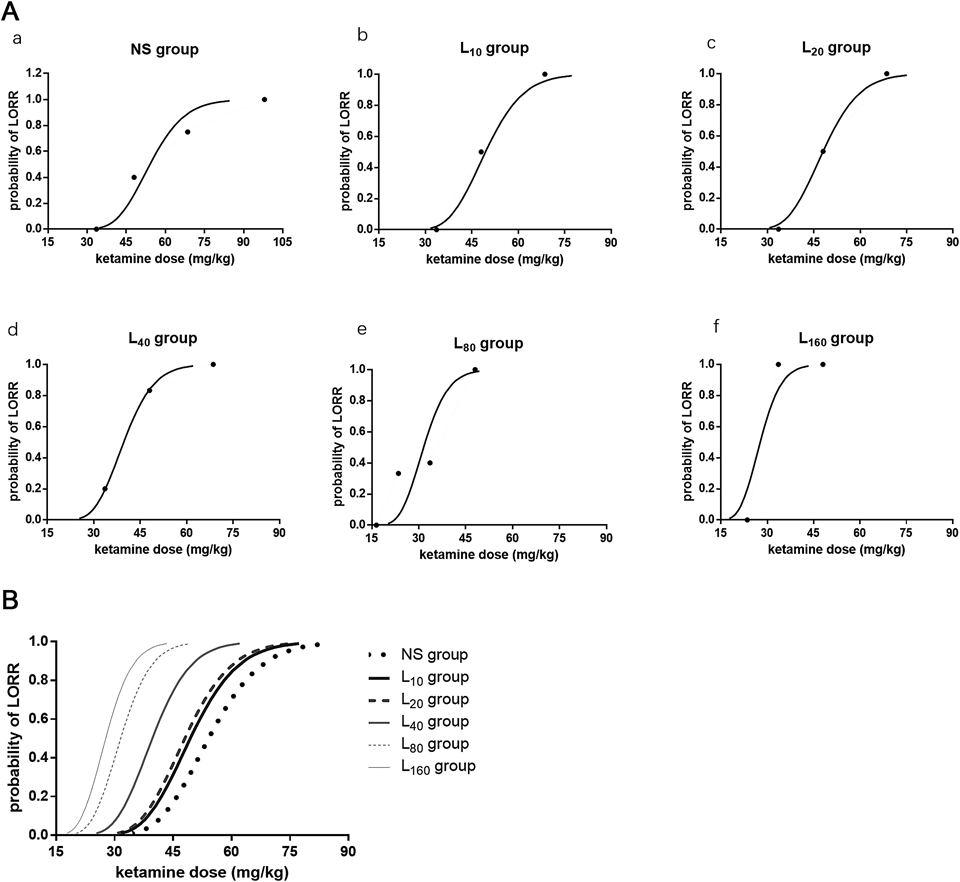
Ligustrazine or normal saline were administered 15 min before ketamine. The dose of ketamine-induced LORR was determined via up-and-down sequential method. (A): dose–response curve of each concentration of ligustrazine. (B): summarized dose–response curves of different dose of ligustrazine. Each color represented one concentration. NS: normal saline; Ket: ketamine; L10: ligustrazine 10 mg·kg−1; L20: ligustrazine 20 mg·kg−1; L40: ligustrazine 40 mg·kg−1; L80: ligustrazine 80 mg·kg−1; L160: ligustrazine 160 mg·kg−1.
| ED50a) (95%CI) | ED50b) (95%CI) | ED95b) (95%CI) | |
|---|---|---|---|
| Ket | 54.1 (38.4–76.1) | 54.1 (44.8–65.3) | 74.2 (62.0–100.4) |
| L10+Ket | 50.1 (41.1–63.1) | 49.4 (41.4–58.3) | 67.8 (56.9–91.9) |
| L20+Ket | 48.0 (37.2–61.9) | 48.0 (39.7–58.1) | 65.9 (54.9–89.4) |
| L40+Ket | 43.1 (33.8–55.1) | 39.7 (32.9–47.4) | 54.4 (45.8–72.6) |
| L80+Ket | 31.7 (21.8–46.1) | 31.6 (26.2–38.1)* | 43.3 (36.1–58.8)* |
| L160+Ket | 28.1 (23.7–33.4)* | 27.7 (22.6–33.7)* | 38.0 (31.6–51.3)* |
Data are expressed as ED50 (95%CI). LORR: loss of righting reflex; Ket: ketamine; L10: ligustrazine 10 mg·kg−1; L20: ligustrazine 20 mg·kg−1; L40: ligustrazine 40 mg·kg−1; L80: ligustrazine 80 mg·kg−1; L160: ligustrazine 160 mg·kg−1. a) Dixon and Massey up-and-down method. b) Profit analysis; Compared with Ket group, * p<0.05.
The tail-flick latency of mice in seven groups before and after medicine was shown in Table 2. The results of repeated measures ANOVA indicated that test time had significant effects on tail-flick latency (F=8.517, p<0.001). Medicine (ketamine or ligustrazine) also had significant effects on tail-flick latency (F=8.574, p<0.001). However, there was no cross interaction between time and medicine (F=1.223, p=0.222). The baseline values of tail-flick latency showed no significant difference among all the groups. Compared to baseline values, tail-flick latency did not change over time in normal saline group and 160 mg·kg−1 ligustrazine group, while administration of ketamine or the combination of ketamine and ligustrazine increased tail-flick latency of mice 5 and 15 min after injection (p<0.05). Compared to control mice, 160 mg·kg−1 ligustrazine alone did not increased the pain threshold of mice, administration of ketamine or the combination of ketamine and ligustrazine increased tail-flick latency of mice 5 min after injection (p<0.05). Combination of ketamine and 160 mg·kg−1 ligustrazine prolonged the analgesia time from 5 to 30 min. Moreover, mice treated with the combination of ketamine and 160 mg·kg−1 ligustrazine had a longer tail-flick latency than mice treated with ketamine alone at 5 min and 15 min after injection (5 min: 4.64±1.36 vs. 3.79±0.99 s, p<0.05; 15 min: 4.85±0.87 vs. 3.93±0.62, p<0.05).
| Group | Tail-flick latency at different time | ||||
|---|---|---|---|---|---|
| Base value (s) | 5 min (s) | 15 min (s) | 30 min (s) | 60 min (s) | |
| NS+NS | 2.68±1.16 | 2.95±0.81 | 3.31±0.83 | 2.65±0.93 | 2.97±1.16 |
| L10+NS | 2.96±0.76 | 2.69±0.74 | 2.34±0.74 | 3.02±1.31 | 3.05±1.26 |
| NS+Ket | 2.82±0.95 | 3.79±0.99*☆ | 3.93±0.62☆ | 3.15±1.14 | 3.20±0.51 |
| L20+Ket | 2.84±0.87 | 3.86±0.91*☆ | 3.97±0.66☆ | 3.44±0.87 | 3.31±0.89 |
| L40+Ket | 3.29±0.99 | 3.95±0.63*☆ | 3.93±0.97 | 3.73±0.54* | 3.46±0.62 |
| L80+Ket | 2.97±0.79 | 3.89±0.66*☆ | 4.18±1.24☆ | 3.51±1.17 | 3.54±1.19 |
| L160+Ket | 3.43±0.72 | 4.64±1.36*#☆ | 4.85±0.87*☆ | 3.91±1.41* | 3.41±0.75 |
Data are expressed as mean±SD. NS: normal saline; Ket: ketamine; L10: ligustrazine 10 mg·kg−1; L20: ligustrazine 20 mg·kg−1; L40: ligustrazine 40 mg·kg−1; L80: ligustrazine 80 mg·kg−1; L160: ligustrazine 160 mg·kg−1. Compared with base value, ☆p<0.05; Compared with NS group, * p<0.05; Compared with ket group, # p<0.05.
The present study demonstrated that the ED50 of ketamine to induce LORR is reduced after administration of ligustrazine. Ligustrazine increased hypnotic effect of ketamine in a dose-dependent manner. Moreover, ligustrazine at a large dose also increased the analgesic effect of ketamine. These results indicate that ligustrazine strengthens the hypnotic and analgesic roles of ketamine.
Ketamine is a dissociative anesthetic and widely used in the patients for short-term surgery and in the emergency departments because of its rapid sedation and analgesia.32) Researches have demonstrated that ketamine can also act as an analgesic to alleviate perennial postoperative pain or chronic cancer pain.4–6,33) Multiple receptors and channels including NMDA, gamma absorptiometry aminobutyric acid (GABA), opioid and nicotinic acetylcholine receptors, and neuronal Na+ and Ca2+ channels may be involved in ketamine-induced sedation and analgesia.34–36)
The hypnotic and analgesic properties of general anesthetic may be modulated at different, independent sites. LORR is often used to evaluate anesthetic potency to induce hypnosis in animal studies.37) GABA is the major inhibitory neurotransmitters in the brain. Endogenous GABA can induce LORR in a brain concentration-dependent manner.38) Intravenous anesthetic, like pentobarbital and propofol, or inhalational anesthetic increases the sensitivity of the GABAA receptor to GABA, thus induces LORR.39) NMDA receptor itself may not influence LORR. However, it can modulate anesthetics-induced LORR.40) It has been reported that selective NMDA receptor channel blocker MK-801 alone did not induce LORR, while low-dose of MK-801 increases the duration of LORR induced by the intravenous anesthetics in mice.40) Therefore, ketamine-induced LORR may be associated with both activating GABAA receptors and blocking NMDA receptors. In the present study, we found that the ED50 value for ketamine to induce LORR was 54.1 (38.4, 76.1) mg·kg−1 in mice, which is similar to the value from probit analysis 54.1 (44.8–65.3) mg·kg−1. The ED50 value in our study was a little lower than that reported for mice in the literature that is 61 mg/kg.40) The difference of results may be due to the different methods used for measurement. We used the up-and-down sequential method to determine the ED50. Compared with other methods, the precision of results is improved because of the reduced mean squared error.
Spinal cord is the primary site for mediating pain response or immobility.41) Radiant heat-induced tail-flick test is often used to evaluate analgesic potency in animal. The NMDA receptor in the spinal cord dorsal horn is essential for central facilitation of pain transmission produced by peripheral injury, such as inflammatory nociception or neuropathic pain.42,43) In the ketamine-induced analgesia or immobility, opioid receptors, nicotinic acetylcholine receptors and neuronal Na+, Ca2+ channels may exert more important roles.34–36,44) Moreover, blockage of NMDA receptor or activation of GABAA receptor may enhance ketamine-induced analgesia or immobility by stimulating or modulating those molecular targets.33)
Ligustrazine has been widely used as a treatment of cerebrovascular and cardiovascular diseases in clinic in China.45) Furthermore, several animal and cellular studies have demonstrated that ligustrazine plays protective roles in the ischemic brain injury or learning and cognitive dysfunction of Alzheimer’s disease (AD) model in mice.46) The possible protective mechanisms of ligustrazine include inhibitory effect on the calcium overload, anti-apoptotic activity, anti-inflammatory potential and attenuation of oxidative damage.47–49) The effects of ligustrazine on ketamine-induced hypnosis and analgesic potency have not been studied before. Our results demonstrated the mice received ligustrazine in advance require significantly lower ED50 and ED95 concentrations of ketamine to lose their righting reflexes, suggesting that these mice are more sensitive than the control mice in ketamine-induced hypnosis. Ligustrazine decreased the concentrations of ketamine-induced hypnosis in a dose-dependent way in mice and shifted the dose–response curve to the left in a parallel manner (Fig. 1). Ligustrazine at 160 mg·kg−1 also enhanced ketamine-induced the analgesic effect as reflected by the increase of the tail-flick latency induced by heat radiation in mice. More importantly, ligustrazine prolonged the duration of analgesic effect induced by ketamine from 5 to 30 min. Although different anatomic sites may be involved in the general anesthetic-induced analgesia and hypnosis,41) ligustrazine had effects on both of them indicate that ligustrazine may influence the sites which analgesia and hypnosis are associated with. One hundred sixty milligrams·kilogram−1 ligustrazine alone (the largest dose) neither caused mice LORR, nor increased the pain threshold of mice indicated the effects of ligustrazine on ketamine-induced hypnotic and analgesic roles should be synergistic action.
The cellular and molecular mechanisms of ligustrazine on ketamine-induced hypnosis are still unclear. Glutamate is the major excitatory neurotransmitters in the brain that activates NMDA receptors in the postsynaptic membrane.50,51) Ligustrazine is reported to decrease the concentration of glutamate by inhibiting its biosynthesis and secretion as well as enhancing its uptaking.26) Therefore it is possible that ligustrazine inhibits glutamate neurotransmission by reducing glutamate level. As we mentioned above that blockage of NMDA receptors indirectly enhanced anesthetic-induced analgesia and hypnosis. The enhancement of ligustrazine on ketamine-induced analgesia and hypnosis may be related to glutamate inhibition or reduction of NMDA receptors activity. Since inhibition of Ca2+ channels activation is also involved in ketamine-induced analgesia, ligustrazine attenuated [Ca2+]i by supppressing Ca2+ influx26,52) may also contribute to its synergistic effect on ketamine-induced analgesia. The glutamate level, [Ca2+]i and NMDA receptors activity in the mouse brain need to be measured after ligustrazine administration to reveal these mechanisms in the future studies.
In clinical practice, ligustrazine used alone did not exert any hypnotic effect in patients. Although our present results showed that ligustrazine enhanced the hypnotic and analgesic effect of ketamine in mice, whether it has the same effect in human remained unknown. In the rat models of neuropathic pain53,54) and trigeminal neuralgia,55) ligustrazine showed inhibitory effects on pain transmission. Our present results did not find ligustrazine alone have analgesic roles. It is possible that the mice did not suffer chronic neuropathic pain in the present experiments and that the pain transmission was normal. These studies suggested ligustrazine can elevate the pain threshold of mice with peripheral injury while have no influence on normal pain threshold.
In summary, we found that ligustrazine strengthens the hypnotic role of ketamine at a low dose and promotes the analgesic effect of ketamine at a large dose in mice. These results suggest that some Chinese medicine may exert influence on anesthetic potency of ketamine. Since ligustrazine is widely used for patients with cerebrovascular and cardiovascular diseases in China, it is important to study the effects of ligustrazine on ketamine or other anesthetics in human.
This work was supported by the National Natural Science Foundation of China (81371259, 81641160); the Natural Science Foundation of Guangdong Province, China (2016A030313251); the Science and Technology Planning Project of Guangdong Province, China (2014A020212147) and the Science and Technology Planning Project of Guangzhou, China (201605122118121) the Medical Science and Technology Program of Foshan, China (20130841).
The authors declare no conflict of interest.