2018 Volume 41 Issue 5 Pages 707-712
2018 Volume 41 Issue 5 Pages 707-712
Oridonin, the major terpene found in Rabdosia rubescens, is widely used as dietary supplement or therapeutic drug, while the effects of oridonin on CYP450 were still unclear. The pregnane X receptor (PXR) is an important regulatory factor for major drug metabolism enzyme CYPs, and it has been reported to have species-specific differences. Therefore, this study has employed more reliable models PXR-humanized mouse to investigate the influence of oridonin on PXR and downstream metabolism enzyme. Eight-week-old male PXR-humanized mice were treated with oridonin by orally (0, 25, 50, 100, 200 mg/kg) for 15 d. The effects of oridonin on major downstream CYPs of PXR were examined at both the mRNA and enzyme activity levels by RT-PCR and HPLC-MS/MS. In general, there was no significant toxic reaction in liver of PXR-humanized mice. The mRNA expression of CYPs and cytochrome P450 oxidoreductase (POR) were increased with oridonin treatment in a dose-dependent manner. CYP2c and CYP3a family catalytic activity were increased significantly in two higher doses groups. These results indicate that oridonin induced the expression and activation of CYP2c and CYP3a family, which might contribute to potential drug–drug interactions and appear to be a risk when co-administered with other clinical drugs.
Traditional herbal medicines derived from plant are widely used as supplements or therapeutic drugs worldwide. However, the metabolic characteristic of herb or herb–drug combinations is often unpredictable, which largely limited the extensive application of traditional herbal medicine. Case reports, clinical trials, and in vitro studies have shown that there are significant risks of interactions between herbal medicines and drugs by inducing or inhibiting drug metabolizing enzymes.1,2) Therefore, the herb–drug interactions are urgently needed to investigate to instruct the treatment based on herbal medicines better.
Oridonin is a diterpenoid extracted from Rabdosia rubescens and widely used as a dietary supplement or therapeutic drug3) (Fig. 1). Oridonin has been linked with the suppression of survival, proliferation, invasion and angiogenesis of cancer. Many studies have demonstrated that oridonin can induce apoptosis in a variety of cancer cells including those from prostate, breast, non-small cell lung cancers, acute leukemia, glioblastoma multiforme, and human melanoma cells.4–8) The results indicated that oridonin is a potential anti-tumor agent in future anticancer therapeutics and should be co-administered with other chemotherapy drugs. Meanwhile, the use of oridonin with prescription drugs is on the rise and gaining increasing popularity, the risk of adverse interactions increases which remains poorly recognized. However, whether oridonin could affect the metabolic process of its co-administered drugs by influencing metabolic enzymes is still unknown.
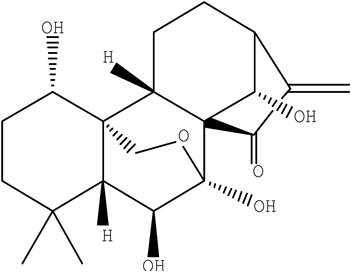
((14R)-7-Alpha,20-epoxy-1-alpha,6-beta,7,14-tetrahydroxykaur-16-en-15-one, C20H28O6, CAS No.: 28957-04-2).
The CYPs constitute the major enzyme family capable of catalyzing the oxidative biotransformation of most drugs or other xenobiotics, and are therefore of particular relevance to clinical pharmacology.9,10) There is a unique combination of mechanisms and regulation factors such as xenobiotics, cytokines and hormones to influence the expression of CYPs through the nuclear receptors mediated pathways. Many compounds had been reported to regulate CYPs. For example, Ginkgo biloba extract up-regulated CYP3A4 mRNA and CYP3A-mediated testosterone 6β-hydroxylation via affecting nuclear receptor. It indicated that nuclear receptors play an important role in herbal medicine activation of CYP in drug metabolism.11) In our previous studies, we have employed the reporter gene assays and found that oridonin can induce the expression and activity of CYP3A4 through nuclear receptor pregnane X receptor (PXR) by directly binding to the base pairs in CYP3A4 promoter.12)
The PXR (NR1I2) is a member of the nuclear receptor family. The characteristic structural features of PXR include a highly-conserved DNA-binding domain (DBD), which links the receptors to specific promoter regions of their target genes involved CYP3A, CYP2C family and transporters, and a less conserved ligand binding domain (LBD) that permits them to directly interact with hormones or xenobiotics.13–15) The ability of xenobiotics to activate PXR is depending on species. For example, rifampicin is a strong human PXR-specific activator while it has a very weak effect on mouse PXR. Compared to more than 90% amino acid identity in DBD, the LBD of human and mouse PXR only share 76% identity. The sequence divergence among species is considered to be responsible for the species-specific PXR activation. It is well-known that the antibiotic rifampicin is a specific PXR activator in humans, but has no effect on PXR which is a homologs gene of human PXR in mice.16) Meanwhile, pregnenolone-16α-carbonitrile (PCN) is a specific PXR agonist in mice.17) All these observations may limit the extrapolation from mice to human, and difficult to anticipate the response of herbal medicine. In our previous studies, we have tried to evaluate the effects of oridonin on PXR activation and CYP450 enzymes genes expression in C57BL/6 mice, while the wild-type mouse models are highly unreliable predictors to evaluate the human CYP450 response in herb–drug interactions due to the species-specificity of PXR response. To avoid the species differences in induction, Xie et al. used PXR-humanized mice which knocking-out the rodent PXR gene and replacing it with the human PXR to establish a ‘humanized’ mouse model, were proved useful in predicting and avoiding drug–drug interactions.18,19) In this study, we used the humanized PXR mice as a quantitative model to predict human CYPs induction.
In the present study, we evaluated the effects of oridonin on major CYP450s genes expression and activation in PXR-humanized mice and provided at least part of the basis for herb–drug interactions which could be the guidance of drug metabolism in oridonin and chemotherapeutics combination.
Oridonin (purity >99%) was purchased from Chinese National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Hydroxytolbutamide, 1′-hydroxymidazolam and 7-hydroxycoumarin were supplied by Toronto Research Chemicals (North York, Ontario, Canada). TaKaRa Biotechnology Co., Ltd. (Dalian, China) provided primeScripts RT reagent Kit With gDNA Eraser and SYBRs Premix Dimer Eraser™, and Sigma-Aldrich (St. Louis, MO, U.S.A.) offered corn oil and other chemicals used in this study.
Animals and TreatmentsPXR-humanized mice (23±2 g, 8-weeks old) were kindly provided by Professor Xie Wen from University of Pittsburgh. All mice were maintained under a standard 12-h dark and 12-h light cycle with temperature-, light-, and humidity-controlled environment and treated with oridonin at a dose of 0, 25, 50, 100, 200 mg/kg (dissolved in corn oil) once a day for 15 d. Livers were collected and frozen in liquid nitrogen, and stored at −80°C before use. All animal care and experimental procedures were carried out according to the guidelines for the care and use of laboratory animals issued by the Animal Center of the Central South University and approved by the Independent Ethics Committee Institute of Clinical Pharmacology, Central South University. The bioethical allowance number is CTXY-140003-17.
HistopathologyAfter 15 d treatment, livers of PXR-humanized mice were first removed, weighed, and fixed in 10% neutral buffer formalin to hematoxylin–eosin (H.E.) staining for histological analysis. Representative microscopic images were taken for histologic analysis to comparison were from the same portion of the liver from each animal.
Blood BiochemistryThe serum activities of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were detected by Medsoul AMS-224 analyzer (Medsoul Instruments, Inc., Bejing, China) to examine the potential hepatotoxicity of animals treated with the various doses of oridonin.
Total RNA Extraction and Real-Time PCR QuantificationTotal liver RNA was extracted using the TRIzol reagent and quantified with the absorbance at 260 nm. The optical density (O.D.) 260 nm/O.D. 280 nm ratio was measured to assess the purity of RNA, and the formaldehyde-agarose gel electrophoresis was used to evaluate the integrity of RNA.
Reverse transcription of RNA to cDNA was performed on Prime Scripts RT reagent Kit. Primers for related genes were chosen from previous studies and the mRNA expression was normalized against β-actin.20) PCR assays were quantified using Light Cyclers 480 (Roche Diagnostics, Penzberg, Germany) per manufacturer’s protocol.
Determination of Liver Microsomal CYPs ActivitiesLiver microsomes from each mouse were prepared following the method of Umegaki et al.21) Microsomal protein concentration was performed using Pierces BCA Protein Assay Kit (Thermo Scientific, Rockford, IL, U.S.A.) per manufacturer’s instructions. These microsome samples were stored at −80°C before analysis. Liver microsome suspension and nicotinamide adenine dinucleotide phosphate (NADPH) regeneration system solution used Prime TOX™ (Novca Bio-Technology, Guangzhou, China) were placed in microtubes and incubated at 37°C. Each enzymatic reaction was performed in triplicate under the conditions according to Zhang et al.22) The supernatant was prepared for HPLC-MS/MS analysis of CYP450-enzyme activities. Chromatographic and MS conditions were optimized as previously described.23–25) Chromatographic separations were used a Waters Acquity BEHC18 column (2.1×50 mm, particle size 1.7 µm, Waters, Wexford, Ireland) with an isocratic elution. Mobile phase was water with 0.1% formic acid/acetonitrile (70/30) at a flow rate of 0.2 mL/min. MS analysis was performed in selected reaction monitoring (SRM) mode with an electrospray ionization (ESI) probe in positive-ion mode. The details about assay methods are shown in Table 1. To detect the metabolite, mass chromatograms and mass spectra were acquired by MassLynx software (Waters, Milford, MA, U.S.A.).
| CYP3a | CYP2c | |
|---|---|---|
| Probe drug | Midazolam | Tolbutamide |
| Concentration (µM) | 50 | 100 |
| Liver microsomes (µg) | 25 | 30 |
| Assay volumes (µL) | 150 | 150 |
| Precursor ion (m/z) | 342 | 287 |
| Qualifier (m/z) | 324 | 171 |
| Collision energy (eV) | 20 | 15 |
| Metabolites | 1′-Hydroxymidazolam | 4-Hydroxytolbutamide |
All quantitative data are expressed as mean±standard deviation (S.D.). Statistical analysis was carried out by means of one-way ANOVA using GraphPad 5 (GraphPad software Inc., La Jolla, CA, U.S.A.). For all analyses, values of p<0.05 were considered statistically significant.
Histopathology and serum activities of ALT, AST were examined to evaluate the liver function of PXR-humanized mice treated with various doses of oridonin. Representative histopathology histopathological images are shown in Fig. 2. No significant morphological alterations were observed in liver sections from PXR-humanized mice (Fig. 2B–E) as compared to control (Fig. 2A). Meanwhile, according to biochemical analysis, the ALT and AST activities of PXR-humanized mice treated with oridonin in serum is similar to control group (Fig. 3).

For each group, images show representative foci of control (A), 25 mg/kg (B), 50 mg/kg (C), 100 mg/kg (D), and 200 mg/kg (E) groups. H.E. staining. Magnitude (400×). Liver sections of mice treated with oridonin showed largely normal appearance.

All values are presented as mean±S.D.
The mRNA expression levels of CYPs are shown in Fig. 4. As we can see from Fig. 4, the gene expression of CYP2c family was most markedly and dose-dependently increased than the control group after oridonin treatment. The highest dose group (200 mg/kg) increase the mRNA expression levels of CYP2c37, CYP2c39 and CYP2c40 by 3.78±0.63-, 3.37±0.60- and 2.15±0.24-fold (p<0.01), respectively. The mRNA level of CYP3a11, a homologous gene of CYP3A4, was increased by 3.14±0.49-fold (p<0.01) in 200 mg/kg oridonin-treated groups. However, there are no significant cytochrome P450 oxidoreductase (POR) expression changes for all treated groups, which indicated that the POR as the sole electron donor for all CYPs change might adapt to the enhanced CYPs expression.

All values are presented as mean±S.D.; * p<0.05, ** p<0.01 (n=6).
The activities of CYPs enzyme were detected by the metabolite of the specific probe drug after oral-treatment with various concentrations of oridonin for 15 d. As shown in Fig. 5, significantly higher CYP2c and CYP3a catalytic activities were observed in 100 and 200 mg/kg oridonin-treated groups than other two lower dose groups. CYP2c activity was up-regulated by 2.56±0.55- and 3.58±0.88-fold (p<0.01) in 100 and 200 mg/kg oridonin-treated groups, respectively. While CYP3a activity was increased by 1.89±0.29- and 2.18±0.54-fold (p<0.01) in 100 and 200 mg/kg oridonin-treated groups, respectively.

All values are presented as mean±S.D.; * p<0.05, ** p<0.01 (n=6).
The PXR-humanized mice are classical panel of mouse models to evaluate the role of human pregnane X receptor in drug response. Replacing mouse PXR with the human homologous gene makes the possibility of using humanized mice as a quantitative model to predict human CYP3A4 induction.18) The use of PXR-humanized mouse model to accurately quantify the impact of rifampicin-mediated CYP3a11 induction on RNA expression, enzyme activity, and triazolam pharmacokinetics in vivo, while rifampicin could not induce the CYP3a11 in wild-type mice.26) Here we examined the effect of oridonin in CYPs activation on humanized PXR mice, the availability of such model is particularly valuable during the early stages of compound selection and lead optimization, as they can provide a quantitative measure of the magnitude of a drug–drug interaction.
In the present study, we treated humanized PXR mice with a wide range of oridonin orally 0, 25, 50, 100, 200 mg/kg for 15 d. This range of doses employed according to the average amount of oridonin contained in Rabdosia rubescens, an herbal medicine currently used in China for human consumption. The animal dose calculated directly from a 60-kg human dose with the standard animal/human km value, which is published in the U.S. Food and Drug Administration (FDA)’s guidance. According to histopathological and serological examination, there is no obvious liver damage observed in humanized PXR mice after oridonin treatment, which is consistent with our previous study that oridonin was well tolerated in mice.22) In previous reports, rifampin, a positive control for CYP3a, could induce hepatotoxicity.27) In consideration that these prototypical inducers could cause some pathologic changes, the induction potential of oridonin was compared with only the control. Then, we systematically examined the induction effects of oridonin on major hepatic drug metabolizing enzymes CYPs and POR at their mRNA levels in humanized PXR mice. The results indicated that oridonin has markedly induced transcriptional activity of 3a11 and 2c family. The enzyme activities of CYP2c, 3a families exhibit a statistically significant increase, which consistent with the up-regulation of mRNA level. These results indicated that oridonin has the potential to cause pharmacokinetics interactions with other clinical drugs leading to either increased or decreased plasma levels of clinical drugs, and could result in unexpected toxicities or reduced efficacy.
Oridonin is one such agent which has attracted much attention and research.28) The present study offers the pharmacological basis for the animal experiments and initial clinical application of oridonin which can significantly suppress tumor growth and induce synergistic effect with chemotherapy drug such as idarubicin which metabolized by CYP2C9, and it also has reported that oridonin combined with imatinib which metabolized by CYP3A4 exerted synergetic suppressive effects in Ph+ acute lymphoblastic leukemia cells.29,30) The present study may provide at least part of the evidence for oridonin–drug interactions for the up-regulation of oridonin on the hepatic drug metabolizing system.
In conclusion, the present study found markedly inductive effects of oridonin on hepatic drug metabolizing enzymes mRNA expression, as well as enzyme activity in humanized PXR mice. Therefore, oridonin should be used with caution to avoid potential herb–drug interactions, although it is safe with high dose in vivo. Clinical studies on the pharmacokinetic profile of drugs co-administered with oridonin, relative to their induction of CYPs needs to be further explored. We hope that our study will contribute to better utilize and combine herbal preparations with other drugs so that patients can use them with minimum risk and optimum benefit.
This study was financially supported by the National Natural Science Foundation of China (No. 81503165), Natural Science Foundation of Zhejiang Provincial (No. Q17H310010), Science and Technology Planning Project of Administration of Zhengjiang Province Traditional Chinese Medicine (No. 2015ZA038).
The authors declare no conflict of interest.