2018 年 41 巻 5 号 p. 811-814
2018 年 41 巻 5 号 p. 811-814
In previous studies we showed that the complexation hydrogels based in poly(methacrylic acid-g-ethylene glycol) [P(MAA-g-EG)] rapidly release insulin in the intestine owing to their pH-dependent complexation properties; they also exhibit a high insulin-loading efficiency, enzyme-inhibiting properties, and mucoadhesive characteristics. Cell-penetrating peptides (CPPs), such as oligoarginines [hexa-arginine (R6), comprising six arginine residues], have been employed as useful tools for the oral delivery of therapeutic macromolecules. The aim of our study was to investigate the combination strategy of using P(MAA-g-EG) hydrogels with R6-based CPPs to improve the intestinal absorption of insulin. A high efficiency of loading into crosslinked P(MAA-g-EG) hydrogels was observed for insulin (96.1±1.4%) and R6 (46.6±3.8%). In addition, immediate release of the loaded insulin and R6 from these hydrogels was observed at pH 7.4 (80% was released in approximately 30 min). Consequently, a strong hypoglycemic response was observed (approximately 18% reduction in blood glucose levels) accompanied by an improvement in insulin absorption after the co-administration of insulin-loaded particles (ILP) and R6-loaded particles (ALP) into closed rat ileal segments compared with that after ILP administration alone. These results indicate that the combination of P(MAA-g-EG) hydrogels with CPPs may be a promising strategy for the oral delivery of various insulin preparations as an alternative to conventional parenteral routes.
Multiple subcutaneous (s.c.) injections of insulin remain the common approach for the treatment of insulin-dependent diabetics.1) However, the s.c. insulin injection cannot mimic the physiological hypoglycemic mechanism of insulin. In addition, the pain and inconvenience of multiple daily injections often result in poor patient compliance.2,3) Such incidents have led to numerous efforts toward the development of a safe and effective noninvasive insulin delivery system.
In the last 25 years, oral delivery of insulin has been widely investigated to minimize the risks of s.c. insulin injection and improve the QOL of diabetics.4) However, oral delivery of insulin remains a great challenge because of low stability and poor membrane permeability of insulin in the gastrointestinal tract.5) So far, several encapsulation techniques, including liposomes and polymeric nanocarriers have been developed, however, their drug incorporation efficiency is generally very low as well as the bioavailability has not been sufficient to develop oral formulations of insulin.
In a previous study,6) we developed pH-responsive polymeric hydrogels for the oral delivery of insulin, hydrogels which comprised crosslinked poly(methacrylic acid) grafted with poly(ethylene glycol) [P(MAA-g-EG)].6) The crosslinked P(MAA-g-EG) hydrogels rapidly release insulin in the intestine owing to their pH-dependent complexation properties; they also exhibit a high insulin-loading efficiency, enzyme-inhibiting properties, and mucoadhesive characteristics without adversely affecting the integrity of the intestinal epithelial membranes.7,8) Although these properties are quite favorable for oral peptide delivery, still they have limited success in achieving acceptable oral insulin bioavailability.
Recently, we reported that cell-penetrating peptides (CPPs), such as oligoarginines, can enhance permeation for the oral delivery of therapeutic macromolecules.9) We demonstrated that the co-administration of oligoarginines, such as hexa-arginine (R6, a peptide comprising six residues of arginine), significantly improved the intestinal absorption of therapeutic peptides and proteins poorly absorbed in the gastrointestinal tract without affecting the epithelial cellular integrity.9) Based on these findings, it was hypothesized that if P(MAA-g-EG) hydrogels can effectively incorporate as well as release the CPPs and insulin, they can improve the oral bioavailability of insulin. In this study, we examined the usefulness of the combination strategy with P(MAA-g-EG) hydrogel and the oligoarginine R6 in improving the intestinal absorption of insulin. We investigated the loading and release properties of insulin and R6 using P(MAA-g-EG) hydrogel. Furthermore, we evaluated the absorption of insulin after the co-administration of insulin- and R6-loaded P(MAA-g-EG) hydrogels (ILP and ALP, respectively) into in situ ileal loop.
P(MAA-g-EG) microparticles were synthesized by the UV-initiated free-radical solution polymerization of methacrylic acid (MAA, Sigma-Aldrich Co., St. Louis, MO, U.S.A.) and tetraethylene glycol dimethacrylate (TEGMA, Polysciences Inc., Warrington, PA, U.S.A.) with poly (ethylene glycol) (PEG, molecular weight of 1000), as described previously.10) The loading of human recombinant insulin (Wako Pure Chemical Industries, Ltd., Osaka, Japan) and R6 (L-form, Bachem AG, Bubendorf, Switzerland) into the hydrogels was performed by equilibrium partitioning as described previously.11) In brief, 0.5 mg/mL insulin and 5.2 mM R6 solutions were prepared in phosphate-buffered saline (PBS, pH 7.4) containing 0.001% methylcellulose, which prevents the adsorption of insulin on the tube surface. Loading was accomplished by imbibing 10.5 mg and 7 mg of dried P(MAA-g-EG) microparticles in 1.5 mL of insulin and 1 mL of R6 solution, respectively, with stirring for 2 h. The particles were collected by centrifugation (at 6600 rpm, 2 min), dried under vacuum, and stored at 4°C before use. The loading efficiency of insulin into P(MAA-g-EG) was determined by HPLC as described before,10) and the loading efficiency of R6 into P(MAA-g-EG) was determined using a BCA protein assay kit (Thermo Fisher Scientific Inc., Waltham, MT, U.S.A.). Insulin- and R6-loaded P(MAA-g-EG) particles were designated as ILP and ALP, respectively. The incorporation/adhesion ratio was calculated using the following equation.
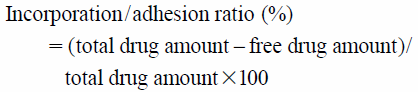 |
In total, 4 mg of ILP or 2 mg of ALP hydrogel were added to 20 mL or 1 mL of PBS (pH 7.4) containing 0.001% methylcellulose, respectively, and stirred at 37°C. Samples were collected at discrete intervals until 180 min, and the same volume of PBS was added to maintain the volume of the release media. The concentration of insulin and R6 was determined as described above. The fractional release of insulin and R6 from the formulations, defined here as the ratio of the amount released at any time, Mt, to the total amount released after 3 h, M∞, was calculated.
In Situ Loop Absorption ExperimentsThe animal experiments conducted in this study complied with the regulations of the Committee on Ethics in the Care and Use of Laboratory Animals at Hoshi University. Male Wistar rats weighing 170–210 g were fasted for 24 h before the experiments and anesthetized by an intraperitoneal injection of 50 mg/kg sodium pentobarbital (Dainippon Sumitomo Pharmaceutical Co., Ltd., Osaka, Japan). The rats were restrained in a supine position on a thermostatically controlled board at 37°C.
Following a small midline incision made carefully in the abdomen and its proximal-to-ileocecal junction segment (length, 10 cm) was exposed and cannulated at both ends using polypropylene tubing. These segments were securely ligated to prevent fluid loss and carefully returned to their original location inside the peritoneal cavity. After 1 h of rest, ILP microparticles or ILP with ALP microparticles suspended in 1.5 mL of PBS solution were directly administered into the 6-cm ileal loop made from the 10-cm pretreated segment. The dose for insulin was fixed at 20 IU/kg and 2.5 mg of ALP was administered to each rat. During the experiment, a 0.2-mL blood aliquot was withdrawn from the jugular vein at t=0, 5, 10, 15, 30, 60, 120, and 180 min after dosing. The plasma was separated by centrifugation at 13000 rpm for 1 min. Blood glucose concentration was measured with a glucose meter (Novo Assist Plus, Novo Nordisk Pharma Ltd., Tokyo, Japan).
The plasma insulin concentration was determined using an enzyme immunoassay kit (Insulin ICMA kit, Molecular Light Technology, Wales, U.K.). The area under the curve (AUC) was calculated using the trapezoidal method based on plasma insulin concentration–time profile, and the area above the curve (AAC) was derived from successive trapezoids of the blood glucose concentration–time profile. The relative bioavailability (BA) and pharmacological availability (PA) of administered insulin were calculated relative to the s.c. route, which indicated the magnitudes of insulin absorption and hypoglycemic reaction, respectively. In brief, an insulin solution was prepared by dissolving an appropriate amount of recombinant human insulin in PBS at an s.c. dose of 1.0 IU/kg body weight. To maintain the same physical conditions for the rats, the same surgery (ileal loop) was performed on animals receiving s.c. insulin as on rats in the intestinal absorption study.
Statistical AnalysisEach value is expressed as the mean and standard error of the mean (S.E.M.) of multiple determinations. The significance of the differences in the mean values between groups was evaluated using ANOVA with Student’s unpaired t-test. Differences were considered significant when the p value was less than 0.05.
Our recent study demonstrated that the intestinal absorption of insulin can be significantly improved by the co-administration of CPPs, such as oligoarginines, in a physical mixture.9) In the noncovalent CPP strategy for enhancing oral absorption of macromolecules, we demonstrated that an intermolecular interaction between CPPs and macromolecules was essential to initiate the action of CPPs. In our next step, it will be required to establish the clinical dosage forms appropriate for the noncovalent CPP strategy. In our previous study, P(MAA-g-EG) hydrogel improved the intestinal absorption of therapeutic peptides and proteins by protecting loaded insulin in the stomach, and their extensive release in the small intestine.12) In addition, P(MAA-g-EG) hydrogel microparticles inhibit gastrointestinal proteolytic enzymes and have intestinal mucoadhesive properties.7,8) Therefore, we considered it valuable to investigate the potential of the hydrogel as a delivery carrier of insulin and CPPs.
In this study, the incorporation ratios of insulin and R6 into P(MAA-g-EG) hydrogel were 96.1±1.4% (n=6) and 46.6±3.8% (n=5), respectively. The results of insulin-loading efficiency experiment showed that insulin could be significantly incorporated into hydrogels, which was consistent with the results of our previous report.11) R6 was also effectively loaded into the hydrogel and retained by it; however, this value was lower than that of insulin. In our previous study, P(MAA-g-EG) hydrogel demonstrated a high capacity for incorporation of macromolecular bioactive agents, highlighting the possibility that R6 could also be efficiently loaded into the hydrogel.10) The relatively lower loading efficiency of R6 may be due to its lower molecular weight (insulin: 5800 Da vs. R6: 960 Da) and structural differences. In particular, R6 has a simple arginine chain structure, while insulin may have a bulkier structure. These results suggest that P(MAA-g-EG) hydrogel has a specifically high affinity for peptide drugs.
In this study, the release characteristics of insulin and R6 loaded in hydrogels were examined. Insulin and R6 were immediately released from the hydrogels at pH 7.4 (Fig. 1), and approximately 80% of the loaded insulin and R6 was released in approximately 30 min. Our previous study demonstrated that the intermolecular interaction between insulin and oligoarginine is a driving force for the stimulation of intestinal insulin absorption through a noncovalent CPP strategy.13) Therefore, the rapid release of insulin and R6 appears to be ideal for the instant formation of molecular complexes of both molecules, which contributes to the enhancement of the oral absorption of insulin. P(MAA-g-EG) hydrogel has the ability to control insulin release from it in response to changes in environmental pH.14) Thus, these results suggest that P(MAA-g-EG) hydrogel microparticles are an appropriate tool for delivering insulin and R6 following oral administration.

Each data point represents the mean±S.E.M. (n=5–6).
Figure 2 shows the plasma concentration profile of insulin (Fig. 2A) and the resultant hypoglycemic effect (Fig. 2B) following ILP administration with or without ALP into the ileal segments. An insignificant hypoglycemic response was observed following the administration of ILP (Fig. 2B), which demonstrated that insulin was not absorbed by the intestinal segments (Fig. 2A). In contrast, the co-administration of ILP with ALP enhanced the ileal absorption of insulin (Fig. 2A), which suggested that the absorption of insulin released from ILP was increased by the effect of R6 simultaneously released from ALP. Blood glucose levels decreased to approximately 82% of their initial value after the administration of ILP with ALP in contrast to those after the administration of ILP alone (Fig. 2B). Table 1 summarizes the pharmacokinetic parameters derived from the insulin concentration–time profiles following in situ administration of ILP with or without ALP into the ileal segments. The parameters Cmax, AAC, AUC, PA, and BA after co-administration of ALP with ILP increased compared with those after the administration of ILP alone. Although the increases were statistically insignificant due to large inter-individual variations (p=0.0615), the PA value of ILP co-administered with ALP was considerably higher than that of ILP administered alone. In addition, the BA value of ILP co-administered with ALP was clearly higher than that of R6 administered with insulin in solution.9) These results imply that as expected from the release study results, P(MAA-g-EG) hydrogel can provide an ideal environment for in vivo intermolecular interactions between insulin and oligoarginine. These promising data of our pilot study may be useful in further investigations for the development of oral delivery systems for therapeutic peptides and proteins using various CPPs as potential enhancers and P(MAA-g-EG) hydrogel as a delivery carrier. However, in the preliminary experiment when oligoarginine and insulin were incorporated simultaneously, the incorporation ratios of both molecules significantly decreased. Therefore, in this study, insulin and R6 were separately incorporated into the hydrogel microparticles. Considering the molecular complex formation efficiency after release from the microparticles, drug and CPPs should be present in the same formulation. To utilize P(MAA-g-EG) hydrogel microparticles as a delivery carrier in the noncovalent CPP strategy, further studies are required to optimize the formulation.

Each data point represents the mean±S.E.M. (n=5–6).
| Cmax (µU/mL) | Tmax (min) | AAC (% glu·reduc·h) | AUC (µU·h/mL) | PA (%) | BA (%) | |
|---|---|---|---|---|---|---|
| ILP | 20.2±10.7 | 35.0±17.5 | 2.3±2.3 | 17.0±8.1 | 0.2±0.2 | 0.8±0.4 |
| ILP+ALP | 188.4±101.5 | 11.0±1.0 | 34.2±16.3 | 177.6±127.2 | 3.0±1.4 | 9.1±6.5 |
Each value represents the mean±S.E.M. (n=5–6). Cmax, maximum concentration; Tmax, time to reach Cmax; AAC, area above the curve; AUC, area under the curve; PA, relative pharmacological availability compared with s.c.; BA, relative bioavailability compared with s.c.
In this study, we demonstrated that P(MAA-g-EG) hydrogel microparticles have a high loading efficiency for insulin and the model CPP, R6, which are effectively released in the intestinal environment. Furthermore, we observed that the co-administration of ILP and ALP into the intestine improved the absorption of insulin and reduced blood glucose levels. These findings suggest that the combination of P(MAA-g-EG) hydrogel carriers contributing to protection and controlled release of drugs and CPP with permeation stimulatory effect can be an effective strategy for the oral administration of insulin.
The authors declare no conflict of interest.