2019 Volume 42 Issue 11 Pages 1898-1905
2019 Volume 42 Issue 11 Pages 1898-1905
Pharmaceutical applications of three dimensional (3D) printing technology are increasing following the approval of 3D-printed tablets by the U.S. Food and Drug Administration. Semi-solid extrusion-type 3D printers are used to 3D print hydrogel- and paste-based materials. We previously developed tablet formulations for semi-solid extrusion-type 3D bioprinters. In the present study, we extended our study to the preparation of muco-adhesive oral film formulations to 3D bioprint mouth ulcer pharmaceuticals. We focused on hydroxypropyl methylcellulose (HPMC)-based catechin (model drug)-loaded hydrogel formulations and found that the viscosity of a hydrogel formulation is dependent on the HPMC concentration, and that viscosity is important for facile 3D printing. HPMC-based films were prepared using two different drying methods (air drying and freeze drying). The films exhibited different drug dissolution profiles, and increasing the amount of HPMC in the film delayed drug dissolution. The fabrication of HPMC-based catechin-loaded films with different shapes provides a model of individualized, on-demand pharmaceuticals. Our results support the flexible application of 3D bioprinters (semi-solid extrusion-type 3D printers) for preparing film formulations.
Three-dimensional (3D) printing technologies are currently finding applications in medicine.1,2) Rapid prototyping is a common use of 3D printing to develop approaches for efficiently designing and manufacturing parts such as jigs and entire devices. Various medical devices3,4) and imaging phantoms5) have been produced using 3D printers. 3D printing of a surgical site is useful for understanding the pathology of the site before surgery and for aiding patient understanding of their condition prior to giving consent for surgery. The internet site ClinicalTrial.gov (URL: https://clinicaltrials.gov/) has registered many clinical trials related to “3D printing.” Most clinical trials involving 3D printing of surgical guides and anatomical models currently aim to facilitate the planning of musculoskeletal surgery and oral/maxillofacial surgery,6) although several recent clinical trials have involved the 3D printing of implants for addressing bone defects and breast reconstruction for individual patients. 3D printing of cells (bioprinting) also holds promise for patient-specific tissue fabrication as a regeneration therapy.7,8) The contribution of 3D printing technology to personalized medical therapy is thus clearly expanding.
New pharmaceutical applications of 3D printing technology are increasing following the U.S. Food and Drug Administration approval of the manufacturing of a 3D printed tablet (SPRITAM) in August 2015.9,10) This orodispersible tablet is manufactured in bulk using a powder-based 3D printer and ZipDose technology. Other types of 3D printers using various printer materials have been studied to explore novel applications of 3D printed pharmaceuticals. For example, Khaled et al. reported the preparation of a polypill (a multiple active pharmaceutical tablet) using a multi-nozzle-equipped semi-solid extrusion type 3D printer.11,12) Additionally, tablets with unique designs and geometries prepared by 3D printing allow controlled drug release.13–16) Polypills and novel tablets manufactured using a 3D printer may allow personalized dosing, an important advancement since each patient has unique pharmacokinetics and metabolism. The use of 3D printers for preparing pharmaceutical doses may change both drug manufacturing and therapeutic approaches.
We previously reported the preparation of tablets using a pressurized air semi-solid extrusion-type 3D bioprinter to extrude bio-ink.17) 3D bioprinters have been studied for possible applications in tissue engineering, for example by using hydrogel/paste-based materials to prepare cell-incorporated bio-inks. The manufacture of skin and body parts using a 3D bioprinter holds promise for future transplantation therapy,18) and 3D printing of organs-on-a-chip (e.g., liver) that can replicate organ-level functions which would transform the screening and testing of drugs.19,20) Pharmaceutical applications of 3D bioprinters may allow the preparation of temperature-sensitive drugs and biopharmaceuticals by using a hydrogel/paste-based drug formulation17) because bioprinters do not require high temperature, in contrast to typical 3D printers (e.g., the conventional fused deposition modeling (FDM)-type 3D printer used to prepare tablets).
The aim of this study was to extend the application of tailored 3D-printed pharmaceuticals to muco-adhesive oral films composed of hydroxypropyl methylcellulose (HPMC) using catechin as a model drug. This novel film formulation would treat inflammations of the oral cavity such as aphthous stomatitis and oral mucositis ulcers. Aphthous stomatitis is a common mucosal disorder caused by biting, acidic foods, smoking, and various immune reactions due to systemic and viral diseases.21) Oral mucositis ulcers occur in cancer patients following radiation therapy and chemotherapy.22,23) Catechins are flavonoids and polyphenols found in tea leaf and exhibit anti-oxidant, anti-inflammatory, anti-cancer, and anti-hypertensive effects.24) The use of catechin-based films may help alleviate oral ulcers when combined with standard treatments.
Muco-adhesive buccal films and orodispersible films are attracting considerable interest as new oral formulations for local and systemic drug administration.25) The properties of HPMC-based muco-adhesive films prepared by traditional casting methods have been reported,26,27) and the preparation of oral films with different doses and shapes is important for personalized dosing and therapy. Film formulations have been prepared using inkjet-type and flexographic-type 2D printers28–30) and a FDM-type 3D printer,25,31–33) whereas to our knowledge there have been no reports describing the preparation of drug-loaded oral muco-adhesive film formulations using a 3D bioprinter. Here we characterized printer-inks (HPMC-based hydrogels containing a drug) and the resulting films (dried 3D-printed films).
HPMC (METOLOSE 90SH-15000SR HPMC 2208 type) was supplied by Shin-Etsu Chemical Company, Ltd. (Tokyo, Japan). D(−)-Mannitol, Tween 20, Tween 80, glycerol and ethanol were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). (+)-Catechin hydrate was purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.).
Preparation of Hydrogel-Based Printer InksHPMC hydrogel-based drug formulations were prepared as printer inks as previously reported, with modification.34) Water (7 mL) and a magnetic stirrer were added in a screw-top glass vial and placed in a water bath at 80°C, then appropriate amounts of HPMC powder and mannitol were added to the vial with stirring. Catechin (50 mg) was dissolved in 3 mL ethanol/water (2/1, v/v), heated to 80°C and added to the vial, followed by 40 µL each Tween 80 and glycerol at 80°C and the solution was stirred well. The drug formulations are described in Table 1. The vial was cooled and kept at room temperature overnight to remove bubbles.
| (A) | |||||||
|---|---|---|---|---|---|---|---|
| Formulation | HPMC (mg) | Catechin (mg) | Mannitol (mg) | Water (mL) | Ethanol (mL) | Tween (mL) | Glycerol (mL) |
| A | 100 | 0 | 200 | 8.0 | 2.0 | 0.040 | 0.040 |
| B | 200 | 0 | 200 | 8.0 | 2.0 | 0.040 | 0.040 |
| C | 300 | 0 | 200 | 8.0 | 2.0 | 0.040 | 0.040 |
| D | 400 | 0 | 200 | 8.0 | 2.0 | 0.040 | 0.040 |
| E | 500 | 0 | 200 | 8.0 | 2.0 | 0.040 | 0.040 |
| (B) | |||||||
| Formulation | HPMC (mg) | Catechin (mg) | Mannitol (mg) | Water (mL) | Ethanol (mL) | Tween (mL) | Glycerol (mL) |
| A′ | 100 | 50 | 150 | 8.0 | 2.0 | 0.040 | 0.040 |
| B′ | 200 | 50 | 150 | 8.0 | 2.0 | 0.040 | 0.040 |
| C′ | 300 | 50 | 150 | 8.0 | 2.0 | 0.040 | 0.040 |
| D′ | 400 | 50 | 150 | 8.0 | 2.0 | 0.040 | 0.040 |
| E′ | 500 | 50 | 150 | 8.0 | 2.0 | 0.040 | 0.040 |
The viscosity of each sample was measured using a rotational cone-plate viscometer (Brookfield viscometer DV2T, Brookfield, Middleboro, MA, U.S.A.) with a CPE-40 or CPE-52 spindle (Brookfield) at 25°C. The rotation speed was gradually increased (0.1, 0.2, 0.5, 1, 2, 5, 10, 20, 50, 100 and 200 rpm) every 30 s, and the shear stress and viscosity were measured.
3D Design and Printing, and Preparation of the FilmsFilm-like sheets (40 × 40 × 0.35 mm) was designed using 123D Design 3D CAD software (Autodesk Inc., San Rafael, CA, U.S.A.) (Fig. 1A). The 3D printing conditions were set using Slic3r slicer software (GNU General Public License) (Fig. 1B). The printer ink prepared as described in previous section was carefully loaded into a syringe with a 27G nozzle, then set in the 3D bioprinter (INKREDIBLE; CELLINK; Gothenburg, Sweden). A clear polypropylene sheet was placed on the 3D printer stage to support film-like hydrogel sheets printed by the extrusion of printer ink from the nozzle (Fig. 1C). The extrusion of ink was controlled by using the air pressure through pump (Formulation B, 20 kPa; Formulation C, 50 kPa; Formulation D, 70 kPa, as a typical experiment). The printed hydrogel was air dried at room temperature (air drying method, AD method) and stored in a desiccator. Alternatively, the hydrogel was frozen at −80°C and freeze-dried using a FD1000 freeze-dryer (EYELA, Tokyo, Japan) (freeze drying method, FD method).

(A) 3D design of a film sheet using 3D CAD software. (B) 3D printing program settings. (C) 3D printed film sheet. (Color figure can be accessed in the online version.)
The weights of film samples after drying were measured on an electric balance. The thicknesses of film samples were measured at five randomly selected points using a digital micrometer to the nearest 0.001 mm (MDC-25MX, Mitutoyo, Kawasaki, Japan).
Dissolution TestDissolution tests of the film formulations were conducted using paddle-type methods according to Japanese Pharmacopoeia 17th edition and a reference.35) The dissolution vessel was placed in the dissolution tester (NTR-6200 A; Toyama Sangyo, Osaka, Japan) and 300 mL 0.1% Tween 20 was added. The temperature of the solution was maintained at 37°C and the stir rate was 50 rpm. Films prepared in previous section were folded, individually inserted into a metal sinker to prevent the sample from floating, then placed in the vessel. Aliquots of sample solution were collected at appropriate time points and the same volume of 0.1% Tween 20 solution was injected. The concentration of drug in solution was determined by measuring the absorbance of each aliquot at 280 nm using an UV visible spectrometer (UV1800; Shimadzu, Kyoto, Japan).
Scanning Electron Microscopy (SEM)The film samples were observed by SEM (S-4300, Hitachi; Tokyo, Japan) after coating with Pt–Pd using an ion sputtering apparatus (E-102; Hitachi).
Powder X-Ray DiffractionFilm and powder samples were analyzed by powder X-ray diffraction measurements using a Rint-Ultima (Rigaku Co., Ltd., Tokyo, Japan) by irradiating with Cu-Kα X-rays. The tube voltage and amperage were 44 kV and 40 mA, respectively. Samples were scanned between 2θ of 3 to 45°.
We first prepared drug-loaded hydrogel formulations as 3D bioprinter inks. The film formulations consist of an active pharmaceutical ingredient, water soluble polymer, sweetening agent, surfactant, plasticizer, and other components (e.g., saliva stimulating agent, flavors, colors, fillers).36) The drug formulations are described in Table 1. HPMC, mannitol, Tween, and glycerol were used as the water-soluble polymer, sweetening agent, surfactant, and plasticizer, respectively.
The viscosities of drug formulations with different concentrations of HPMC (Formulation A′–E′) were measured because the polymer concentration greatly affects viscosity, which in turn affects extrusion of the printer-ink from the nozzle. Printer-ink with a low viscosity can leak from the nozzle while ink with a high viscosity is difficult to handle and extrude. Consequently, the viscosity of the ink must be regulated to prepare pharmaceutical-quality film formulations using an extrusion-type 3D bioprinter.
The rheological properties of the drug-loaded printer-inks are shown in Fig. 2. The rheogram (relationship between shear stress and shear rate) changed as the concentration of HPMC changed (1–5%, Formulation A′–E′). The formulation with the lowest HPMC concentration (Formulation A′) provided an almost linear relationship (Fig. 2A), indicating Newtonian flow, as well as the lowest viscosity of the five formulations (Fig. 2F) which resulted in its leakage from the bioprinter nozzle. In contrast, the formulation with the highest HPMC concentration (Formulation E′, Figs. 2E, F) was difficult to extrude from the nozzle. Formulation B′–D′ appeared suitable for 3D printing through the 27G nozzle. The particle size of hydrophobic drugs and excipients in formulations must be controlled to prevent nozzle clogging. The HPMC-based hydrogel formulations prepared in the present study are clear and dissolved and thus suitable as printer-inks for extrusion-type 3D printers.

(A–E) Formulation A′–E.′ (F) Viscosity–shear rate data. The data show the means ± standard deviation (S.D.) (n = 3).
The rheograms of these formulations are shown in Figs. 2C and D and indicate that the formulations are stable against stress which are lower than yield value (stress). Higher stress can result in material flow. The application of strong air pressure can cause the printer ink to extrude and the printed hydrogel retains its shape without collapsing under its weight. We believe that the viscosity properties of our formulations are useful for 3D printing, as supported by our previous 3D bioprinting of tablets.17) The viscosity characteristics of formulations without drug (Formulation A–E) are shown in Supplementary Fig. 1 and are very similar to the corresponding drug-containing formulation (Formulation A′–E′), showing that the viscosity of the printer ink was dependent on the concentration of HPMC.
Film Preparation by Drying 3D Printed Hydrogel Formulations and Film CharacteristicsFilm-like hydrogel sheets were 3D printed (Fig. 1C) and the HPMC-based films were obtained by drying, as shown in Fig. 3. Two types of films were prepared using two drying methods. The first was the simple AD method (Figs. 3A, C, E). Increasing the amount of HPMC resulted in the formation of suitable clear films (Fig. 3E) whereas films with insufficient HPMC were white, indicating crystallization of the mannitol and drug. Films formed using formulation B′-AD were very scratch-resistant (Fig. 3A). In the present study, 50 mg catechin was incorporated in the hydrogel stock formulation (Table 1B). The increase of catechin may have the possibility to crystallize the drug and to affect the appearance of air-dried film. We also prepared films using the FD method (Figs. 3B, D, F) and obtained cotton-like fine films using all three formulations. The weights and thicknesses of films prepared by the two methods are shown in Table 2. The weights of the films were similar using both methods but the thicknesses varied greatly, with FD samples being almost 10-times thicker than AD samples due to differences in the preparation method.

(Color figure can be accessed in the online version.)
| (A) | |||
|---|---|---|---|
| Formulation | Weight (mg) | Drug (mg) | Thickness (µm) |
| B′-AD | 50.9 ± 9.0 | 5.2 ± 0.9 | N.D. |
| C′-AD | 40.5 ± 3.3 | 3.4 ± 0.3 | 38 ± 6 |
| D′-AD | 46.7 ± 7.1 | 3.4 ± 0.5 | 33 ± 3 |
| (B) | |||
| Formulation | Weight (mg) | Drug (mg) | Thickness (µm) |
| B′-FD | 51.0 ± 11.6 | 5.2 ± 1.2 | 398 ± 49 |
| C′-FD | 33.9 ± 3.5 | 2.9 ± 0.3 | 358 ± 73 |
| D′-FD | 45.7 ± 7.5 | 3.3 ± 0.6 | 352 ± 43 |
The data show the means ± S.D. (n = 5).
The in vitro dissolution profiles of drugs from the films are shown in Fig. 4. The B′-AD film was difficult to scratch, and thus, we tested two AD formulations (Formulation C′-AD and D′-AD) and three FD formulations (Formulation B′-FD, C′-FD, D′-AD). Formulation D′-AD and D′-FD exhibited delayed drug dissolution, suggesting that gel formation around the surface of these HPMC-based films controlled drug release. The drug release profile of HPMC matrix drug tablets has been investigated in detail.37) Increasing the amount of HPMC in a film delays dissolution, thereby controlling drug dissolution. Additionally, HPMC is adhesive and thus is used as a binder. Moistened HPMC-based film in the oral cavity is likely muco-adhesive and releases drug locally in a controlled manner, depending on the amount of HPMC.
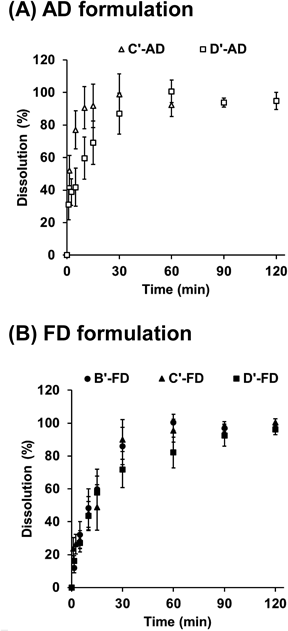
The data show the means ± S.D. (n = 3).
Jamroz et al., prepared aripiprazole-loaded orodispersible film by using FDM-type 3D printer.33) The 3D printing which is additive manufacturing process can produce the pore (about 650 × 300 µm) in the film structure, and the drug dissolution from film was increased compared with the film prepared by conventional casting method due to the increase of surface area. If we produced the film with the pore and lattice by using semi-solid extrusion type 3D printer, it could change the drug dissolution. The high degree of freedom in design is a potential advantage of 3D printing.
We next studied the microstructures of the films by obtaining SEM images (Fig. 5). FD films have porous structures (Figs. 4A, C, E) and AD films do not. The amount of HPMC in the films may affect the porous structure. Films formed using Formulation B′-AD contain low amounts of HPMC and had rough and large pores whereas films formed using Formulations C′-FD and D′-FD contain higher amounts of HPMC and had small pores. These differences in microstructure did not greatly affect drug dissolution (Fig. 4), likely because the simple geometry (film) has a minimum effect on drug dissolution using the current experimental conditions. Drug dissolution from Formulation C′-AD was unexpectedly faster than that from formulation C′-FD (Fig. 4). We had assumed that FD films with porous structures would lead to rapid drug dissolution due to the large surface area, but the opposite results were obtained, perhaps because the different thickness between AD and FD samples affected drug release (FD samples were approximately 10-times thicker, as shown in Table 2). Physical properties were assessed using powder X-ray diffraction (Fig. 6). The strong mannitol and relatively weak catechin peaks in AD and FD formulations (Formulation D′-AD and D′-FD) disappeared due to amorphous state of HPMC.


Formulations D′-AD and D′-FD, described in Table 2, were used as the film samples.
As shown in Fig. 7, various designs and shapes of AD and FD films were designed and prepared. Aphthous stomatitis is typically characterized by small, round or ovoid ulcers38) whereas the shapes of mouth ulcers formed by cheek bites differ depending on the strength of the bite. The shape of oral mucositis induced by radiation therapy and chemotherapy also vary depending on the individual and thus we attempted to prepare individualized films with desired shapes using a semi-solid extrusion-type 3D bioprinter as model 3D printed tailored pharmaceuticals. 3D printed manufacturing of personalized pharmaceuticals was previously proposed.39) An electronic prescription is sent from a hospital department to a 3D printer, and raw materials in the printer ink are used to manufacture formulations (tablets) using a 3D printer under quality control via process analysis technology. Film formulations are likely also applicable to this concept, since 3D bioprinters can handle several types of drug formulations. The present study showed the probable utility of 3D bioprinters in clinical settings.

(A) 3D designs of various film shapes, (B) AD films, and (C) FD films. Formulations D′-AD and D′-FD, described in Table 2, were used as the film samples. (Color figure can be accessed in the online version.)
In conclusion, we focused on identifying appropriate printing conditions for drug formulations of HPMC-based hydrogels by varying the polymer concentration, and hence the viscosity, of prepared AD and FD films. FD films appear to be advantageous for preparing films because all FD films were easy to handle, of the appropriate thickness, and had good visual characteristics. The present study provides useful information on a model pharmaceutical for personalized therapy generated using a semi-solid extrusion-type 3D printer.
This research was partly supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (19K07170).
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.