2019 年 42 巻 3 号 p. 319-326
2019 年 42 巻 3 号 p. 319-326
Ischemic stroke is one of the leading causes of severe disability and death. In clinical settings, tissue plasminogen activator (t-PA) for thrombolytic therapy is the only globally approved drug for the treatment of ischemic stroke. However, the proportion of patients who receive t-PA therapy is extremely limited due to its narrow therapeutic time window (TTW) and the risk of cerebral hemorrhage. Cerebral ischemia–reperfusion (I/R) injury is also a serious problem for patients’ outcomes. Hence, the development of more effective therapies has been desired to prolong the TTW of t-PA and prevent cerebral I/R injury. For delivering drugs into the brain, the blood–brain barrier (BBB) must be overcome since it limits drug penetration into the brain, leading to insufficient therapeutic efficacy. As a distinctive pathology after an ischemic stroke, it was reported that the vascular permeability of the BBB is increased around the ischemic region. We found that nano-sized liposomes can pass through the disrupted BBB and accumulate in the I/R region, and that delivery of neuroprotective agents using a liposomal drug delivery system (DDS) is effective for the treatment of cerebral I/R injury. Moreover, we have recently demonstrated that combination therapy with liposomal drugs and t-PA can suppress the deleterious effects of t-PA and extend its TTW in a rat ischemic stroke model. These findings indicate that applications of nanoparticle DDS technology could be a hopeful approach to drug development for ischemic stroke therapy. In this review, we introduce our findings on ischemic stroke treatment using liposomal DDS and recent advances from other research groups.
Cerebrovascular disorders comprise conditions with the high mortality rates and are a leading cause of long-term disability worldwide.1) Ischemic stroke accounts for about 60% of these disorders, and the rest comprise cerebral hemorrhage and subarachnoid hemorrhage. Ischemic stroke is caused by a reduction of cerebral blood flow due to thrombi and leads to brain cell death in the ischemic region. Around the ischemic core region, there is an area called the ischemic penumbra, which is known to allow reversible escape from cell death by the rapid restoration of blood flow.2) In clinical settings, thrombolytic therapy with tissue plasminogen activator (t-PA), the only globally approved therapeutic agent for acute ischemic stroke, can be administered to salvage brain cells in the ischemic penumbra. However, due to several problems such as a narrow therapeutic time window (TTW) and safety concerns regarding the risk of cerebral hemorrhage, the proportion of patients given t-PA treatment is very low.3) In addition, after reperfusion with t-PA or surgical treatments, cerebral ischemia–reperfusion (I/R) injury often occurs via oxidative stress and inflammation and leads to poor outcomes for patients.4) As a neuroprotective agent against I/R injury, the radical scavenger edaravone (Radicut; Mitsubishi Tanabe Pharma Co., Osaka, Japan) has been approved in a limited number of countries (i.e., Japan, P. R. China, and India),5) and its therapeutic benefit was also reported in Europe.6) However, its clinical application remains limited due to renal disorders reported as serious adverse consequences following treatment with edaravone.7) Hence, the development of more broadly applicable, effective drugs is urgently needed to extend the narrow TTW of t-PA and prevent cerebral I/R injury.
Since multiple mechanisms were reported to be involved in the expansion of I/R injury,8) numerous candidate drugs targeting those mechanisms have been explored. Nevertheless, few have been approved due to their insufficient therapeutic benefit due to limited entry into the brain parenchyma and adverse side effects with nonspecific distribution.9,10) To resolve those problems, we considered that the application of drug delivery systems (DDS), especially liposomes, would be a promising approach since it allows targeting, changes the pharmacokinetics of entrapped drugs, and reduces their adverse effects.11) Doxil, a clinically approved liposome modified with polyethylene glycol (PEG) encapsulating doxorubicin, is used for the treatment of carcinoma such as breast and ovarian cancer.12) In the liposomal formulation, the encapsulation of doxorubicin can reduce its adverse side effects such as cardiotoxicity and increase its therapeutic efficacy via the enhanced permeability and retention (EPR) effect.13)
To achieve efficient delivery of therapeutic drugs into the brain, the blood–brain barrier (BBB) must be overcome, as the BBB is functionally constituted to maintain strict brain homeostasis by restricting the transport of molecules into the brain. However, as a distinctive phenomenon after cerebral I/R, it was reported that the vascular permeability of the BBB is increased around the ischemic region by certain mediators such as reactive oxygen species (ROS), matrix metalloproteinases (MMPs), and inflammatory cytokines.14,15) In cancer therapy, the delivery of anticancer agents with nanoparticles by passing through the spaces between leaky tumor vessels is widely applied for the selective delivery of drugs as described above. By focusing on BBB disruption after an ischemic event, we demonstrated that drug delivery into the ischemic region can be achieved by the use of liposomal DDS via passing through the spaces between the disrupted BBB, similar to drug delivery to tumor tissues utilizing the EPR effect. In this review, we introduce our recent findings on the treatment of ischemic stroke with liposomal DDS and other reports using nanoparticle DDS and describe the potential of DDS technologies for drug development for ischemic stroke therapy.
To confirm the applicability of liposomal DDS for ischemic stroke therapy, we first investigated the cerebral distribution of intravenously injected liposomes in the ischemic region after I/R. For this purpose, transient middle cerebral artery occlusion (t-MCAO) rats prepared using the intraluminal filament method were employed.16) The time course of brain damage was investigated with 2,3,5-triphenyltetrazolium chloride (TTC) staining. Obvious brain damage started to be seen in the ischemic area 3 h after reperfusion (Fig. 1A), and the damage expanded with time.17) Then, fluorescence-labeled 100-nm PEGylated liposomes (PEG-liposomes) were intravenously injected into the t-MCAO rats after cerebral I/R, and their distribution in the brain was observed ex vivo. PEG-liposomes were injected at each time point after the start of reperfusion following 1 h of occlusion, and fluorescence imaging was performed 1 h after the injection. The accumulation of fluorescence-labeled PEG-liposomes was detected in the ischemic side even when the liposomes were injected immediately after reperfusion, and the most abundant accumulation was observed when liposomes were injected 3 h after reperfusion (Fig. 1B). These results suggest that the increased permeability of the BBB around the I/R region starts prior to the appearance of obvious brain damage, and that nano-sized liposomes can pass through the disrupted BBB and accumulate in the I/R region before the progression of brain damage. Moreover, the liposomes injected immediately after reperfusion were retained there at least for 24 h (Fig. 1C). Interestingly, higher fluorescence intensity of the liposomes was detected in the brain sections dissected 24 h after liposomal injection than in those dissected 1 h after (Figs. 1B, C). The data on the brain distribution of [3H]-labeled PEG-liposomes that were intravenously injected immediately after reperfusion also showed greater accumulation in the ischemic side compared with the non-ischemic side 3 h after injection (Fig. 1D). Moreover, their accumulation in the ischemic side was significantly greater than that in the non-ischemic side 6 and 24 h after injection (Fig. 1D). These results suggest that PEG-liposomes gradually reach the brain parenchyma in the I/R region due to the EPR effect, similar to the liposomal accumulation in tumor tissue.18)

(A) Appearance of obvious brain damage 3 h after reperfusion following 1-h occlusion in transient middle cerebral artery occlusion (t-MCAO) rats, an ischemic model rat. Brain slices were stained with 2,3,5-triphenyltetrazolium chloride (TTC) to visualize the damaged region. White areas (indicated by white arrows in the images) show the damaged region, and red areas show the surviving region. (B, C) Accumulation of PEG-liposomes in the I/R region. DiIC18 (DiI)-labeled PEG-liposomes were intravenously injected into t-MCAO rats 0 or 3 h after reperfusion following 1-h occlusion. The rats were then euthanized 1 h after liposomal injection, and brain slices were prepared. Thereafter, the DiI fluorescence in the slices was detected with an in vivo imaging system. For the results in Fig. 1C, the brain slices were prepared 24 h after injection. The right and left hemispheres represent the ischemic and non-ischemic sides, respectively. The gradation bar indicates the relative levels of fluorescence intensity. (D) Quantification of accumulation of PEG-liposomes in the ischemic side. [3H]-labeled PEG-liposomes were intravenously injected into t-MCAO rats immediately after reperfusion. At 3, 6, or 24 h after the injection, the radioactivity of the liposomes in the ischemic and non-ischemic sides of the brain was determined. Data represent mean ± standard deviation (S.D.). * p < 0.05, ** p < 0.01 vs. the corresponding value for the non-ischemic side. Figure 1A was adapted, with permission, from Ishii et al.17) Figures 1B–D were adapted, with permission, from Ishii et al.18)
We also examined the influence of particle size on liposomal accumulation in the ischemic region. The results showed that the accumulation of PEG-liposomes approximately 200 nm in diameter was less than that of 100-nm liposomes, and almost no accumulation of liposomes with an average diameter of greater than 800 nm was observed.19) Similar to these results, other teams also reported that when nanoparticles were locally administered to the brain, those with a diameter of less than 100 nm diffused well in the brain tissue, whereas those with a diameter greater than 200 nm lacked diffusive property.20,21) These findings imply that the regulation of particle size is one important factor in developing DDS agents for the treatment of cerebral I/R injury.
Since PEG-liposomes can accumulate in the I/R region before the progression of brain damage, we then examined the delivery of neuroprotective agents carried by PEG-liposomes from immediately after the start of reperfusion for treating cerebral I/R injury in t-MCAO rats. We first selected asialo-erythropoietin (AEPO) as a neuroprotectant. AEPO was reported to exert neuroprotection via anti-apoptotic effects without any hematopoietic activity.22) Since AEPO exerts its protective effect by binding to EPO receptors expressed on the cellular membranes of neurons and glial cells,23,24) we prepared AEPO-modified PEG-liposomes (AEPO-Lip) by post-insertion of distearoylphosphatidylethanolamine (DSPE)-PEG-AEPO conjugates after preparing the PEG-liposomes (Fig. 2A), by which AEPO on the liposomal surface is expected to bind quickly to those receptors after reaching the I/R region. Although AEPO is known to show low accumulation in the brain due to its short half-life, a biodistribution study using [125I]-labeled AEPO indicated that liposomalization significantly prolongs its blood circulation time and increases the amount accumulated in the ischemic region. Moreover, treatment with AEPO-Lip significantly suppressed brain cell damage as identified by TTC staining in comparison with free AEPO-treated groups 24 h after reperfusion (Fig. 2B). Histological analysis revealed that intravenously administered AEPO-Lip not only leaked from the disrupted BBB but also bound to blood vessels around the I/R region (Fig. 2C) and markedly accumulated around neuronal cells (Fig. 2D). Since it was previously reported that the expression of EPO receptors in neuronal cells and vascular endothelial cells is increased after the onset of ischemic stroke,23) we consider that the modification of nanoparticle DDS with AEPO can allow specific targeting of cerebral vessels and neurons via binding to its receptors.

(A) Scheme of AEPO-modified PEG-liposomes. AEPO modification on the surface of PEG-liposomes was achieved by post-insertion of DSPE-PEG-AEPO conjugates after preparation of PEG-liposomes. (B) For therapeutic experiments, t-MCAO rats were intravenously injected with PBS, AEPO (8 µg/kg), or AEPO-Lip (8 µg/kg as AEPO dosage) immediately after reperfusion. At 24 h after the injection, the cerebroprotective effects of AEPO-Lip were evaluated by TTC staining. (C, D) Histological analysis of the intracerebral distribution of fluorescence-labeled AEPO-Lip in the I/R region. DiI-labeled AEPO-Lip were intravenously injected into t-MCAO rats immediately after reperfusion. At 24 h after injection, their brains were dissected, and 10-µm frozen sections were prepared. Immunostaining for CD31 (C, cerebral vessels) and NeuN (D, neurons) was performed, and then the liposomal distribution was observed by confocal laser scanning microscopy. Green indicates cerebral vessels (C, Alexa Fluor 488) and neuronal cells (D, Alexa Fluor 488), red indicates liposomes (DiI), and blue indicates nuclei (DAPI). Scale bars = 20 µm. (E) Therapeutic effects of AEPO-Lip on motor function disorder were evaluated using a 21-point neuropathological test. Data represent mean ± S.D. (n = 7). # p < 0.05, ### p < 0.01 vs. PBS-treated group; * p < 0.05, ** p < 0.01 vs. AEPO-treated group. Figures 2A and B were adapted, with permission, from Ishii et al.18) Figures 2C–E was adapted, with permission, from Ishii et al.25)
Neuropathological disorders including motor function deficits are major causes of poor prognoses among ischemic stroke patients, and therefore we also evaluated the therapeutic effects of AEPO-Lip on motor function behaviors in t-MCAO rats. The results showed that a single injection of AEPO-Lip significantly improved motor function deficits compared with free AEPO-treated and control groups even 7 d after I/R (Fig. 2E), suggesting that AEPO-Lip should be useful for the treatment of cerebral I/R injury.18,25)
To demonstrate the further applicability of liposomal DDS for treating cerebral I/R injury, we next investigated the usefulness of liposomes encapsulating low molecular-weight drugs instead of AEPO (glycoprotein), as such drugs are generally easier to handle and less expensive. The immunosuppressant FK506 (tacrolimus) has been widely used to prevent allograft rejection following organ transplantation and reported to be a hopeful drug candidate for the treatment of ischemic stroke.26) Liposomalization of FK506 was achieved by entrapping it in lipid bilayer membranes utilizing its high lipophilicity. The treatment of t-MCAO rats with liposomal FK506 markedly suppressed neutrophil infiltration in the diseased region and apoptosis, resulting in a significant reduction in the extent of brain damage induced by cerebral I/R.27) In addition, the liposomes prevented disorders of motor function and cerebral blood circulation at day 7 following I/R and showed potent neuroprotective effects as evaluated by non-invasive imaging with positron-emission tomography.28)
Next, we prepared fasudil hydrochloride, a Rho-kinase inhibitor, encapsulated in PEG-liposomes (Fasudil-Lip) and examined its usefulness for ischemic stroke therapy. Fasudil is clinically used for the treatment of cerebral vasospasm in patients with subarachnoid hemorrhage, and its safety and effectiveness in the treatment of ischemic stroke were reported.29,30) Fasudil can be efficiently encapsulated into liposomes via the remote loading method using an ammonium sulfate gradient between the internal and external water phases of liposomes.31) Intravenous administration of Fasudil-Lip immediately after reperfusion showed a cerebroprotective effect superior to that of free fasudil administration, implying that liposomalization of fasudil could promote its delivery to the I/R region and increase its therapeutic efficacy.
As mentioned above, liposomes encapsulating low molecular-weight drugs such as FK506 and fasudil could increase their therapeutic efficacy against cerebral I/R injury. Because several factors such as ROS, inflammatory cytokines, etc. are known to be involved in the pathological progression of the injury,32,33) optimization of drug release from liposomes may lead to further augmentation of the therapeutic benefits of liposomal formulations. Investigation of the influence of drug release properties from liposomes on therapeutic effects should be interesting for designing more effective liposomal drugs for ischemic stroke therapy.
The effectiveness of liposomal drugs for the treatment of cerebral I/R injury induced after the restoration of cerebral blood flow by thrombolytic therapy with t-PA or surgical treatments is discussed above. In addition to the prevention of cerebral I/R injury, extension of the narrow TTW of t-PA and prevention of its deleterious effects, including an increase in the risk of cerebral hemorrhage, are required for ischemic stroke treatment. Since increased vascular permeability of the BBB was reported to be induced even under cerebral ischemic conditions,15,34,35) intravenously injected liposomes would reach the ischemic region through the residual blood flow in cerebral vessels. Thereafter, the entrapped drugs would exert cerebroprotective effects, resulting in inhibition of the harmful effects of t-PA and extension of its TTW.
Using MCAO rats, we examined the brain distribution of liposomes injected during occlusion. Accumulation of the liposomes in the ischemic region was observed even when the cerebral perfusion was dramatically reduced by MCAO.36,37) Moreover, the administration of liposomal FK506 prior to reperfusion markedly suppressed cerebral I/R injury in t-MCAO rats,38) suggesting that the liposomes could deliver the neuroprotectants to both the ischemic core and penumbra regions even during the ischemic state.
Next, we investigated the usefulness of combination therapy with liposomal drugs and t-PA in MCAO rats prepared by the photochemically induced thrombosis (PIT) method.39) Unlike the intraluminal filament method used in the above MCAO rat experiments, the PIT method can form blood clots in the MCA by a photochemical reaction using the photosensitizer rose bengal, and those clots can be dissolved by t-PA injection, similar to thrombolytic therapy in clinical settings.40) Fasudil-Lip were used as the liposomal agent for combination therapy, since it was earlier reported that plasmin produced by t-PA treatment can activate Rho-kinase, which leads to BBB breakdown and MMP-9-related cerebral hemorrhagic transformation.41) Thus, liposomal delivery of fasudil is expected to prevent t-PA-related adverse effects and prolong its TTW. Before performing combination therapy, the cerebral distribution of PEG-liposomes was observed in the MCAO rats prepared by the PIT method. The accumulation of intravenously injected PEG-liposomes was observed in the ischemic region before the apparent progression of brain damage (Figs. 3A, B) and occurred in a time-dependent manner (Fig. 3C). These results also demonstrated that drug delivery with liposomes into the ischemic region is possible before reperfusion using t-PA.42) In addition, we have recently found that t-PA treatment significantly increases liposomal accumulation in the ischemic region,43) possibly due to the reperfused blood flow and change in the brain microenvironment, such as BBB integrity and the extracellular matrix, induced by t-PA.
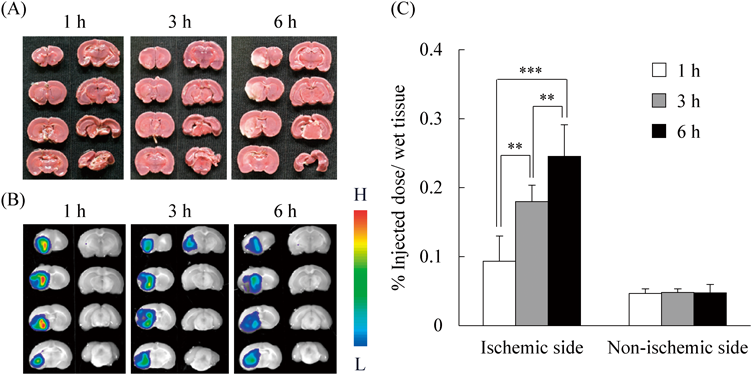
(A) The brains of MCAO rats prepared by the PIT method were dissected 1, 3, and 6 h after the onset of thrombotic occlusion. The time course of brain cell damage was monitored by TTC staining. In this rat model, the left brain hemisphere is the ischemic side, and the right brain hemisphere is the non-ischemic side. (B) DiI-labeled PEG-liposomes were intravenously injected into MCAO rats 1, 3, and 6 h after occlusion. At 1 h after the injection, DiI fluorescence in the brain sections was observed with an in vivo imaging system. The gradation bar shows the relative levels of DiI fluorescence intensity. (C) MCAO rats were injected with [3H]-labeled PEG-liposomes 1 h after occlusion. Radiolabeling of PEG-liposomes was performed with [3H]cholesterylhexadecylether. At 1, 3, or 6 h after liposome injection, the biodistribution of the PEG-liposomes in the ischemic and non-ischemic sides was determined by measuring their radioactivity. Data represent mean ± S.D. (n = 7). ** p < 0.01, *** p < 0.001. Adapted, with permission, from Fukuta et al.42)
For combination therapy, as the TTW of t-PA in MCAO rats was defined as almost 2 h after the onset of occlusion under our experimental conditions, the injection time points of Fasudil-Lip and t-PA were set at 1 and 3 h after occlusion, respectively. The administration of t-PA 3 h after occlusion markedly increased the vascular permeability of the BBB and activated MMP-2 and -9 in the ischemic side, which events are known to be involved in hemorrhage.44) However, combination treatment with Fasudil-Lip prior to t-PA significantly inhibited the permeability increase of the BBB (Figs. 4A, B) and the MMP activation derived from t-PA. These results suggest that fasudil delivered via liposomes may decrease the incidence of hemorrhage after t-PA treatment by inhibiting the Rho-kinase signaling involved in its deleterious effects. In addition, the Fasudil-Lip/t-PA combination treatment significantly reduced neutrophil infiltration into the I/R region (Fig. 4C) and ameliorated ischemic brain damage compared with each treatment alone (Fig. 4D). Importantly, even in the case of t-PA that had been administered at a later time point, i.e., 4.5 h after occlusion, combination treatment showed significantly greater protective effects. These results imply that the combined treatment with Fasudil-Lip could prolong the narrow TTW of t-PA, which was determined to be 2 h after occlusion in MCAO rats prepared using the PIT method, and that combination therapy with liposomal neuroprotective agents plus t-PA could be a useful therapeutic option for the treatment of ischemic stroke.42)

(A) Scheme of liposomal fasudil (Fasudil-Lip). Fasudil was encapsulated into PEG-liposomes using the remote loading method with an ammonium sulfate gradient between the internal and external water phases of liposomes. For combination therapy, the injection time points of Fasudil-Lip and t-PA were set at 1 and 3 h after occlusion, respectively. (B) To evaluate the protective effects of Fasudil-Lip on the t-PA-induced BBB damage, MCAO rats were intravenously injected with Fasudil-Lip (2.5 mg/kg as fasudil dosage) and t-PA (3 mg/kg) as described above, and Evans blue (50 mg/kg in PBS) was also injected 23 h after occlusion. At 24 h after occlusion (1 h after injection of Evans blue), the brains of the rats were dissected, and the amounts of Evans blue accumulated in the brain tissue were measured. Upper panels show representative images of dissected brain sections 1 h after Evans blue injection. Data represent mean ± S.D. (n = 5). * p < 0.05 vs. t-PA-treated group. (C) Infiltration of neutrophils into the I/R region was immunohistologically detected 24 h after occlusion. The black arrows indicate myeloperoxidase (neutrophil marker)-positive cells. Scale bars: 100 µm. (D) The cerebroprotective effects of Fasudil-Lip/t-PA combination therapy were evaluated by TTC staining 24 h after occlusion. Data represent mean ± S.D. (n = 7–8). *** p < 0.001 vs. PBS (t-PA [−]), # p < 0.05 vs. Fasudil-Lip (t-PA [−]) and PBS (t-PA [+]). Adapted, with permission, from Fukuta et al.42)
The delivery of neuroprotective agents using liposome DDS is a hopeful strategy for drug development to treat ischemic stroke, as described above. On the other hand, numerous researchers have recently reported the development of nanoparticle DDS to overcome the BBB and deliver therapeutic agents into the damaged brain after ischemic stroke. Major approaches are the design of active targeting nanoparticles by modifying them with ligands to target molecules expressed around the diseased region. Agulla et al. reported that the heat-shock protein HSP72 is specifically expressed in the peri-infarct region after cerebral ischemia and designed anti-HSP72 antibody-modified immunoliposomes.45) The HSP72-targeting liposomes selectively recognized HSP72-positive cells in vitro and showed higher accumulation in the ischemic side compared with non-modified PEG-liposomes. Moreover, the HSP72-targeting liposomes entrapping citicoline significantly reduced the damaged brain volume as shown in magnetic resonance imaging compared with free citicoline-treated and non-modified PEG-liposomes entrapping citicoline-treated groups. Wang et al. investigated a peptide (HAIYPRH)-modified liposome targeting the transferrin receptor (TfR) loaded with the novel neuroprotectant ZL006.46) Similar to the results of HSP72-targeting immunoliposomes, the TfR-targeting peptide-modified liposomes loaded with ZL006 selectively accumulated in the disease site and showed superior cerebroprotective effects in comparison with other treatment groups without systemic toxicities. From the same research group, Hong et al. reported on their results with dual-targeting liposomes modified with a peptide targeting TfR and stroke-homing peptide (SHp; CLEVSRKNC), which was identified by another research group using an in vivo phage display in a rat model of focal cerebral ischemia.47) The two peptide-modified ZL006-loaded dual-targeting liposomes showed significant accumulation in the ischemic region and inhibited neurological deficits and brain damage compared with each peptide-modified liposome alone.48) Yemisci et al. investigated TfR-1-targeting antibody-modified chitosan nanoparticles carrying basis fibroblast growth factors,49) and Han et al. constructed poly(lactic-co-glycolic acid) (PLGA) synthetic nanoparticles decorated with an MMP-2-targeting peptide, chlorotoxin.50) The PLGA nanoparticles also carry lexiscan, a drug approved by the U.S. Food and Drug Administration for myocardial perfusion imaging, which was earlier revealed to increase the BBB permeability transiently by activating adenosine A2a receptor signaling and to promote drug delivery to the brain.51) These findings demonstrated that the design of active targeting DDS by modification of targeting moieties can efficiently increase the therapeutic benefits of the treatment of ischemic stroke.
As other examples of nanoparticle-based therapy, Hosoo et al. developed a core shell-type nanoparticle containing nitroxide radicals (nitroxide radical-containing nanoparticles [RNPs]).52) The RNPs can scavenge ROS produced following cerebral I/R, prevent BBB damage, and exert neuroprotection in t-MCAO mice. Other teams used red blood cells (RBCs) as a drug delivery carrier. Danielyan et al. found that conjugation of t-PA to RBCs prolongs and localizes the activity of t-PA within the bloodstream and used the conjugate as a thromboprophylactic agent referred to as RBC/t-PA.53) Preinjection of RBC/t-PA brought about rapid lysis of nascent cerebral thrombi, rapid and durable reperfusion, and reduction in morbidity and mortality in a mouse model of cerebral thrombosis caused by injection of preformed fibrin emboli into MCA. Lv et al. developed a bioengineered ROS-responsive nanoparticle using RBC membranes which imparts the natural characteristics of RBCs including biocompatibility, immune-evading ability, and long-term circulating ability in the bloodstream to the nanoparticles.54) The core of nanoparticles is composed of a dextran polymer modified with an ROS-responsive boronic ester which entraps the neuroprotective agent NR2B9C that can selectively inhibit the interaction of N-methyl-D-aspartate receptors (NMDARs) with a postsynaptic density protein (PSD-95).55) By coating the surface of the nanoparticle cores with RBC membranes modified with SHp, the bioengineered ROS-responsive nanoparticles could extend the in vivo circulation time and allow for active targeting to the disease site, resulting in amelioration of neurological disorders and ischemic brain damage in MCAO rats prepared by the intraluminal filament method.
Another interesting approach recently reported is the application of exosomes as nanocarriers for the treatment of ischemic stroke.56) Mesenchymal stromal cell-derived exosomes have the ability to cross the BBB,57) although insufficient targeting capability limits their application as a drug carrier. To improve targeting capability to the BBB, the authors conjugated cyclo(Arg-Gly-Asp-D-Tyr-Lys) peptide (c[RGDyK]) to the exosomes, which shows high affinity to integrin αvβ3 on reactive cerebral endothelial cells after an ischemic event. The cRGD-modified exosomes exhibited significantly higher accumulation in the ischemic brain compared with non-modified exosomes, efficiently entered neurons and glial cells, and inhibited inflammatory responses by encapsulated curcumin. Taken together, applications of the specific properties of circulatory cells or exosomes for drug delivery should also be interesting methods for the development of neuroprotectants for ischemic stroke therapy.
In this review, we described our findings on ischemic stroke therapy using liposomal DDS, as well as recent advances in nanoparticle DDS. Since the permeability increase in the BBB after an ischemic stroke was also reported to occur in human patients,58–60) drug delivery using nanoparticle DDS via the disrupted BBB may be applicable to human stroke therapy. In particular, as shown by our research studies, liposomes can be applied to a lot of neuroprotectants by taking into account their physical properties, including drug candidates that previously failed in clinical trials. Moreover, liposomalization of neuroprotectants can augment their therapeutic benefits by increasing the amounts delivered to the disease sites. Hence, the use of nanoparticle DDS, including liposomes, may increase the success rate of drug development as well as rescue failed drugs in the field of ischemic stroke therapy.
The authors thank all of our collaborators for providing us the materials to perform experiments and the experimental techniques to prepare MCAO models. We also thank colleagues in the Department of Medical Biochemistry, School of Pharmaceutical Sciences, University of Shizuoka. The research performed in our laboratory was supported mainly by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science.
The authors declare no conflict of interest.