2019 年 42 巻 6 号 p. 1030-1033
2019 年 42 巻 6 号 p. 1030-1033
House dust mite (HDM) sublingual immunotherapy (SLIT) in the form of SLIT-tablets is now an established treatment option for HDM allergy and HDM-induced allergic asthma. In SLIT-tablet immunotherapy allergen extracts are formulated as dry tablets and administered under the tongue where it must be solubilized in saliva in order to be able to interact with the immune system of the sublingual mucosa. Solubilization of the extract must occur within a short time span of about one minute after administration, determined by the sublingual holding time recommended by the manufacturer. Currently, two types of HDM SLIT-tablets are available. Both tablet types contain natural HDM extracts from two common HDM species as the active ingredient, but differ with regard to formulation as one tablet type is based on a freeze-dried tablet formulation while the other is based on a compressed formulation. HDM extracts contain a number of major and minor allergens, which in combination provide the allergenic activity that drives the immunological response and in turn the clinical efficacy of the tablets. Here, a biologically relevant human immunoglobulin E (IgE)-based assay is used to compare the ability of the two HDM SLIT-tablet types to deliver HDM allergenic reactivity from the dry tablet into soluble form. The experiments demonstrate that the freeze-dried formulation delivers HDM allergenic activity into solution faster and more efficiently than the compressed formulation.
Allergy immunotherapy (AIT) is currently the only treatment for pollen- and house dust mite (HDM)-induced inhalation allergies which in addition to providing relief of symptoms also has a curative potential with long-term effects that persist after treatment termination. Tablet sublingual immunotherapy (SLIT) is a well-tolerated and convenient route of administration of AIT and is by now the form of AIT with the most comprehensive documentation for clinical effect.1–4) Two different types of HDM SLIT-tablets are currently available; one is based on a fast-dissolving freeze-dried tablet formulation and the other is based on a more conventional compressed tablet formulation. Both contain HDM extracts as the active ingredient, although the major allergen compositions of the extracts differ between the freeze-dried and compressed tablets.3,5,6)
The nominal strengths of all HDM SLIT-tablets registered in Japan are indicated in Japanese allergy units (JAU) established by the Japanese Society of Allergology (JSA).7) The JAU is based on assays developed in Japan by JSA, and provides an accurate measure of the amounts of HDM major allergen contained in each HDM SLIT-tablet.5,7) Additionally, through the use of a common HDM allergen extract standard the nominal strength of JAU-standardized allergen extracts are directly comparable. This is in contrast to other regions, e.g., Europe and Australia, where the nominal strengths of HDM SLIT-tablets are measured with product-specific assays and standards established by the individual manufacturers.8,9)
A particular challenge of SLIT-tablets is that immediately after administration the allergen contained in the tablets must be solubilized in small amounts of saliva in order to become available to the sublingual immune system. Since the assignment of JAU to a HDM SLIT-tablet product is done at the level of the drug substance, i.e., prior to formulation of the allergen extract in tablet form, SLIT-tablets must be able to release the complete allergen content into solution before the full nominal strength of the tablet is delivered to the site of action, the sublingual mucosa.
The efficiency with which HDM major allergens are released into solution by different SLIT-tablet formulations has recently been investigated.5) Major allergen release kinetics is a relevant metric for SLIT-tablet dissolution properties and an accurate marker of drug substance delivery. However, since HDM allergen extracts are known to contain more than 20 different allergens,6) an assay that uses a pool of HDM allergic patients’ immunoglobulin E (IgE) to measure the total allergenic activity of all HDM allergens in combination could be considered as a more biologically relevant method to determine allergen release.
Here we report novel data on the importance of formulation for recovery of the allergenic reactivity of HDM SLIT-tablets, assessed by the use of a biologically relevant, human IgE-based functional assay.
Acarizax (ACZX) (Odactra in the U.S.A.) 20000 JAU/12 SQ-HDM, batch 1464423, expiration date: 03/2017 (ALK-Abello, Denmark) and Miticure (MTC), 3300 JAU, batch 1524573, expiration date: 01/2018 and 10000 JAU/6 SQ-HDM, batch 1524576, expiration date: 01/2018 (Torii Pharmaceutical Co., Ltd., Japan), were obtained from the manufacturer. Actair (ACT) 19000 JAU/100IR, batch 1001-2, expiration date: 05/2018 and 57000 JAU/300IR, batch 3002-1, expiration date: 05/2018, (Shionogi & Co., Ltd., Japan) were obtained through a Japanese pharmacy.
ExperimentsSerum IgE ReactivityAllergenic activity was measured by competition enzyme-linked immunosorbent assay (ELISA): a monoclonal anti-human IgE-antibody (ALK) was immobilized on an ELISA plate and incubated with a serum pool (ALK) obtained from HDM-allergic patients (N = 15, anti-Der p IgE (63.9 KU/L), anti-Der f IgE (51.1 KU/L), anti-Der p 1 IgE (30.9 KU/L), anti-Der f 1 IgE (29.1 KU/L), anti Der p/f 2 IgE (18.6 KU/L)). Unbound human IgE was removed by washing and aliquots of solubilized HDM SLIT-tablet allergen were combined with a biotin-labelled HDM reference extract (ALK) and added to the ELISA plate. Peroxidase-labeled streptavidin (Dako Denmark A/S, Denmark) and 3,3′,5,5′-tetramethylbenzidine (TMB) (Kem-En-Tec Diagnostics A/S, Denmark) was added and the amount of IgE-bound biotinylated HDM extract was determined by spectrophotometry (OD450). The nominal strength of HDM SLIT-tablets in Japan reflects the allergenic potency of the allergen extracts prior to formulation. Strength is assigned in JAU by JSA using JSA-developed assays and an official HDM extract standard.7) Here, the same reagents and HDM standard are used which allows the experimentally determined allergenic potencies of the solubilized HDM SLIT-tablets to be expressed in JAU. Collection of human serum samples with full consent from donors was approved by the local ethical committee. Where used for reference, the total allergenic reactivity of each HDM SLIT-tablet was determined after complete dissolution of the tablets in a test tube.
Dissolution TestRelease Kinetics experiments were performed and test solutions were collected at various time-points as previously reported.5) Briefly, dissolution test was performed according to the paddle method of the Japanese pharmacopoeia using a 200 mL mini vessel (Distek Inc., U.S.A.). Tablets were deposited into 100 mL of assay buffer (100 mM phosphate, pH 6.8, 0.125% casein, 37°C) with 50-rpm paddle speed. Buffer composition is similar to human saliva with regard to pH, ionic strength, and total protein content. The effective allergenic activity of test solutions were measured by ELISA as mentioned above.
All statistical comparisons were performed using GraphPad Prism v.7.02 or SAS v.9.3.
The mean of triplicate (independent) area under the curve (AUC) determinations were compared by one way ANOVA using the Tukey honestly significant difference (HSD) method to correct for multiple comparisons. Normality was assumed for all data. Homogeneity of variance was evaluated by a Brown–Forsythe test.
The drug-substances of the HDM SLIT-tablets were not available for analyses, so the total allergenic potency in each tablet was determined by ELISA following complete dissolution of individual tablets in assay buffer.5) The allergenic potency of Miticure (MTC) 3300 JAU, 10000 JAU and Acarizax (ACZX) 20000 JAU following complete tablet dissolution was experimentally determined to be 3167 ± 167 JAU, 9667 ± 667 JAU and 19667 ± 833 JAU, respectively (mean ± standard deviation (S.D.), n = 3). For Actair (ACT) 19000 JAU and 57000 JAU the measured allergenic potency following complete tablet dissolution corresponded to 8500 ± 333 JAU and 25667 ± 500 JAU, respectively (mean ± S.D., n = 3). As illustrated in Fig. 1, the allergenic reativity released into solution from all three strengths of the freeze-dried HDM SLIT-tablets correspond to the nominal strengths established by JSA. In contrast, the allergenic reactivity released from the compressed HDM SLIT-tablet falls below the line of identity (Fig. 1). This indicates that not all the allergenic reactivity contained in the compressed HDM SLIT-tablets was recovered in soluble form under the conditions used.
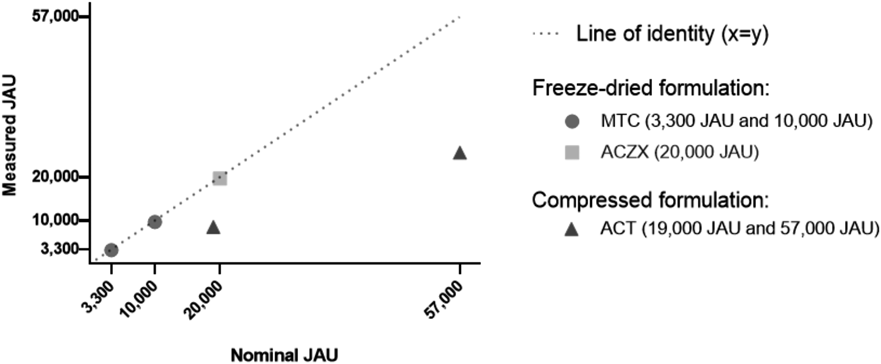
The Line of Identity (x = y) (dashed line) indicates complete correlation between measured and nominal tablet strength.
The allergenic reactivity released over time during tablet dissolution relative to the individual tablets’ total allergenic reactivity is shown in Fig. 2A. The freeze-dried and the compressed HDM SLIT-tablets were examined in parallel under identical experimental conditions. For the freeze-dried HDM-SLIT-Tablets 100% of the allergenic reactivity was released into solution already after 15 s or less (Fig. 2A). In contrast, only a partial release of the total allergenic reactivity was achieved with the compressed tablet (ACT) at any time-point during the experiment, reaching 0.8% at 15 s, 8.8% at 60 s and 33.8% at 300 s (Fig. 2A). With sublingual administration of drugs, the ability to provide fast and complete delivery of the active ingredient (here, HDM allergens), is of essence for maximizing the drug concentration at the surface of the sublingual mucosa. This is due to the phenomenon of “saliva washout” where the drug becomes diluted by saliva after sublingual administration which results in a reduction in the effective potency of the drug.10)
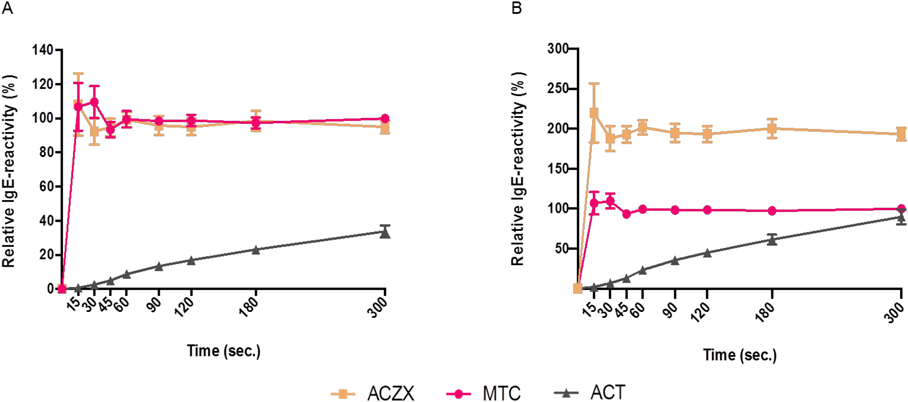
The released IgE-reactivity achieved with MTC (10000 JAU) and ACT (57000 JAU) are statistically significantly different at every time-point (p < 0.05 at t = 300 s (*), p < 0.001 at all other time-points (not shown). (Color figure can be accessed in the online version.)
Figure 2B shows the release of HDM allergenic reactivity from freeze-dried MTC (10000 JAU), ACZX (20000 JAU) and compressed ACT (57000 JAU), with the allergenic reactivity released by MTC as reference. The amounts of HDM allergenic reactivity released by the freeze-dried 20000 JAU ACZX were approximately two-fold higher at all time-points compared to the freeze-dried 10000 JAU MTC, corresponding to the difference in the nominal strengths. In contrast, despite a 5.7-fold higher nominal strength, the allergenic reactivity recovered from the 57000 JAU compressed ACT was below the levels achieved with the 10000 JAU freeze-dried MTC at all time-points (Fig. 2B).
It has previously been suggested that the in-vivo bioavailability of HDM major allergens in SLIT can be expressed as the combination of allergen concentration and mucosal exposure time.5) However, accurate repeated sampling of saliva from human subjects at different time-points starting only seconds after sublingual administration of SLIT-tablets would be very challenging, if at all possible. Instead, the combination of released HDM allergenic activity and time in solution shown in Fig. 2B was used as an in-vitro surrogate parameter for in-vivo allergen bioavailability. This was done by calculating the AUC at each timepoint with the AUCs of MTC (10000 JAU) as reference (Table 1). As expected for tablets with the same formulation, the AUCs of MTC and ACZX are proportional to their respective nominal strengths. In contrast, the relative AUCs obtained with ACT (57000 JAU) increase with dissolution time but remain well below that of MTC at all time-points, even after five minutes in solution.
| Time (s) | Acarizax | Actair | p | Miticure |
|---|---|---|---|---|
| 15 | 2.09 | 0.02 | <0.0001 | 1.00 |
| 30 | 1.95 | 0.04 | <0.0001 | 1.00 |
| 45 | 1.93 | 0.06 | <0.0001 | 1.00 |
| 60 | 1.96 | 0.10 | <0.0001 | 1.00 |
| 90 | 1.98 | 0.17 | <0.0001 | 1.00 |
| 120 | 1.98 | 0.23 | <0.0001 | 1.00 |
| 180 | 1.99 | 0.34 | <0.0001 | 1.00 |
| 300 | 1.99 | 0.51 | <0.0001 | 1.00 |
AUC values are at all timepoints expressed relative to Miticure. p Values indicate the level of statistical significance between the Actair and Miticure AUC at the indicated time points.
It has been claimed by Mascarell and colleagues that allergen bioavailability in SLIT cannot be established on the basis of in-vitro dissolution assays without having demonstrated an appropriate in-vitro and in-vivo correlation.11) However, as suggested by the same group, based on data obtained in an in-vivo model of sublingual immunotherapy, it is in all likelihood possible to reduce the SLIT allergen dose while maintaining efficacy by increasing the amount of allergen in contact with the mucosa and/or increasing the duration of mucosal contact time.12) These data strongly indicate a relationship between the achieved allergen concentration and mucosal contact time and in-vivo efficacy in SLIT, and it furthermore emphasizes that the ability to deliver allergens into solution as efficiently as possible within the recommended sublingual holding time is an essential performance parameter for a SLIT-tablet.
The data presented here demonstrate that only the freeze-dried tablets deliver the entire allergenic activity into solution within one minute, a property that may contribute to the finding that less allergen is required to achieve clinical efficacy with the freeze-dried formulation compared to the compressed formulation (10000 JAU and 57000 JAU, respectively).5,13,14) Consequently, as SLIT-tablet potency standardizations are done at the drug-substance level, and not on the final products, the nominal strengths of HDM SLIT-tablets are only comparable if the tablets are of the same formulation. Since the freeze-dried formulation appears to provide minimal interference between the HDM extract and the tablet matrix resulting in fast and complete allergen-delivery, the freeze-dried formulation seems to be ideal for SLIT-tablet use. Further examination of the physio-chemical properties of the compressed tablet formulation in order to identify the possible interference of individual excipients with allergen delivery would be relevant but is outside the scope of this paper.
We are especially grateful to Dr. Hiroshi Yasueda (Clinical Research Center for Allergy and Rheumatology, Sagamihara National Hospital, Kanagawa, Japan) for helpful suggestions on this research.
This study was conducted by Torii Pharmaceutical Co., Ltd. Katsuyo Ohashi-Doi, Hirokazu Kito, Weibin Du and Hiroshi Nakazawa are employees of Torii Pharmaceutical Co., Ltd., Tokyo, Japan. Kaare Lund is Advisor of Torii Pharmaceutical Co., Ltd., and a shareholder in ALK-Abelló.