2019 年 42 巻 7 号 p. 1102-1111
2019 年 42 巻 7 号 p. 1102-1111
This study aimed to evaluate the effects of combined use of tetrahydrobiopterin (BH4) and nebivolol on cardiac diastolic dysfunction in spontaneously hypertensive rats (SHRs). Twelve-week-old male SHRs were treated with BH4, nebivolol, or a combination of both. Left ventricle function was evaluated, and reactive oxygen species (ROS) production (including dihydroethidium (DHE) and 3-nitrotyrosine (3-NT)), nitric oxide synthase (NOS) activity and the level of NO in myocardial tissue were determined. The expression levels of endothelial NOS (eNOS), phospholamban (PLN), sarcoplasmic reticulum Ca2+ ATPase (SERCA2a), β3-adrenoceptor, cyclic guanosine monophosphate (cGMP), and protein kinase G (PKG) were assayed. Treatment with BH4, nebivolol, or both reversed the noninvasive indexes of diastolic function, including E/E′ and E′/A′, and the invasive indexes, including time constant of isovolumic left ventricle (LV) relaxation (tau), −dP/dtmin, −dP/dtmin/LV systolic pressure (LVSP), and LV end-diastolic pressure (LVEDP) in SHRs. mRNA and protein expression levels of eNOS dimer, phosphorylated PLN, SERCA2a, cGMP, and PKG in the myocardium of treated SHRs were significantly up-regulated compared with those in control rats (p < 0.05 or p < 0.01). The expression levels of 3-NT and DHE were reduced in all treated groups (p < 0.05 or p < 0.01). Notably, combined use of BH4 and nebivolol had better cardioprotective effects than monotherapies. BH4 or nebivolol has a protective effect on diastolic dysfunction in SHRs, and BH4 combined with nebivolol may exert a synergistically cardioprotective effect through activation of β3-adrenoceptor and the NO/cGMP/PKG signaling pathway.
Diastolic dysfunction, characterized by abnormal ventricular diastolic filling, is a prognostic indicator of multiple cardiovascular events.1) Hypertension is supposed to induce diastolic dysfunction via elevated myocardial afterload.2) Oxidative stress may play a critical role in the development of hypertension-induced diastolic dysfunction,3) and the nitric oxide (NO) pathway is closely associated with the level of oxidative stress in patients with diastolic heart failure.4) Multiple studies have demonstrated that decreased NO production is typically related to increased synthesis of reactive oxygen species (ROS) both in patients and animals with heart failure, and NO synthase (NOS) uncoupling has been implicated in this process.5–7) Therefore, increased NO production and inhibition of oxidative stress through NOS recoupling could be an effective strategy to improve hypertension-associated diastolic dysfunction.
Tetrahydrobiopterin (BH4), an important cofactor of NOS, is critical for the synthesis of NO. BH4 deficiency is associated with endothelial NOS (eNOS) uncoupling in vivo, and in this process, eNOS-induced reduction of molecular oxygen leads to the generation of superoxide rather than NO.8,9) In patients with rheumatoid arthritis, both acute and short-term treatment with oral BH4 could improve endothelial dysfunction and decrease the risk of cardiovascular disease.10) BH4 treatment is not only beneficial for increasing the BH4 level in the myocardium and the redox status but also for improving diastolic dysfunction.7,11,12) However, Cunnington et al.13) reported that although BH4 treatment increases total biopterin levels, it has no net effects on the vascular redox state and endothelial function in patients with coronary artery disease. Therefore, a strategy for improving the net action of BH4 on diastolic dysfunction needs to be further elucidated.
β-Adrenoceptor signaling pathways are the primary regulators of cardiac performance.14) Preclinical studies indicated that stimulation of β3-adrenoceptor could further activate eNOS and promote the release of NO.15,16) Nebivolol, the third-generation highly selective β1-adrenoceptor blocker and an agonist of β3-adrenoceptor, has antioxidant and vasodilator effects.17–19) Nebivolol plays critical cardioprotective and antioxidant roles through the β3-adrenoceptor–eNOS pathway16) and exhibits an early improvement of left ventricle (LV) diastolic dysfunction resulting from enhanced NO bioavailability reported in an animal study.20) Based on pharmacological action of BH4 and nebivolol, a new treatment option is urgently needed, and we hypothesize that a combined use may amplify the beneficial effects of BH4 and nebivolol on diastolic dysfunction. Furthermore, whether BH4 and nebivolol have a synergistic effect against diastolic dysfunction and the potential mechanism in this process should be elucidated.
Although a series of studies have been conducted concerning hypertension-induced diastolic dysfunction, there are still no effective treatments for this disease. In this study, we evaluated the effects of BH4, nebivolol, and BH4 combined with nebivolol on the reversal or prevention of diastolic dysfunction in spontaneously hypertensive rats (SHRs).
Forty male SHRs and eight male Wistar-Kyoto (WKY) rats (12 weeks of age) were purchased from Vital River Laboratories (Beijing, China). The animals were fed in a specific pathogen-free facility under the conditions of controlled temperature (23 ± 2°C) and humidity (55 ± 5%) with a 12-h artificial light/dark cycle at the Animal Experiment Center, Gansu College of Traditional Chinese Medicine. The experimental procedures were approved by the Lanzhou University Animal Care and Use Committee. The animal experiments performed in this study conformed the NIH guidelines (Guide for the care and use of laboratory animals) and the guidelines from Directive 2010/63/EU of the European Parliament.
The SHRs were randomly divided into 5 groups (n = 8 per group) as follows: SHR baseline rats did not receive treatment and were fed in the same manner as WKY rats; SHR placebo rats (control) were given saline by gastric gavage; BH4-treated SHRs were given a dose of 25 mg/kg/d BH4 in the same volume of saline as the control group21); nebivolol (N)-treated SHRs were given a dose of 5 mg/kg/d nebivolol in the same volume of saline as the control group22); and N&BH4-treated SHRs were given BH4 (25 mg/kg/d) combined with nebivolol (5 mg/kg/d). The WKY rats served as normal blood pressure controls for SHR baseline rats. At the age of 13 weeks, the rats were given BH4 (Ku Wei Biological Technology Co., Ltd., Guangzhou, China) and/or nebivolol for 1 week.
All of the rats were weighed, and their systolic blood pressure (SBP) and heart rate were measured by a tail-cuff method (Softron BP-98A, Tokyo, Japan) while conscious at 12 and 14 weeks of age. Echocardiography and invasive hemodynamic studies were performed 24–48 h before treatment to obtain baseline data and prior to terminal procedures. Then tissue was harvested for biochemical analysis.
Evaluation of Left Ventricle FunctionSHRs were anesthetized with 2% pentobarbital sodium (0.3 mL/100 g) through intraperitoneal injection. Electrocardiogram (ECG), respiration, and rectal temperature were continuously monitored with an integrated physiology platform (FHC, New Brunswick, ME, U.S.A.). Heart function was evaluated by echocardiography (Vivid E9, GE, Pittsburgh, PA, U.S.A.; Probe i13L Intraoperative Linear Probe). M-mode images in the parasternal long axis and the LV short-axis views at the mid-papillary level were acquired to measure LV posterior wall thickness in diastole (LVPWd), LV end systolic dimension (LVESd), and LV end diastolic dimension (LVEDd). The parameters were averaged from three consecutive beats during expiration. Percent fractional shortening (% FS) and percent LV ejection fraction (% EF) were both recorded.23,24) LV inflow velocities (E and A wave velocities) were measured by conventional pulsed-wave Doppler from the apical four chamber view with the sample volume placed at the tip of the mitral valve leaflets. The mitral annulus longitudinal velocities (E′ and A′) were calculated by pulsed-wave tissue Doppler volume placed at the septal side of the mitral annulus from the apical four-chamber view of the sample.24)
For invasive assessment of heart function, SHRs were anesthetized with pentobarbital sodium and ventilated via tracheostomy. A weight-based algorithm for the selection of tidal volume (0.6–1.25 mL) and respiratory rate (66–114 breaths/min) was performed to keep a minute ventilation volume of 5–10 mL/min (HX-100E, Chengdu Technology & Market Co., Ltd., Chengdu, China). The Mikro-Tip® Pressure Volume (PV) catheter and the MPVS-Ultra™ system (Millar Instruments, Houston, TX, U.S.A.) were used to evaluate LV function in vivo. Body temperature was maintained at 37°C using an electronic animal operating platform (FHC). The PV catheter was inserted in the right common carotid artery and passed through the aortic valve into the left ventricle. Volume and parallel conductance calibration were applied as previously described.24)
Determination of ROS, NO Production and Level of Cyclic Guanosine Monophosphate (cGMP) in MyocardiumFluorescent dye dihydroethidium (DHE, Molecular Probes, Eugene, OR, U.S.A.) was used to determine in situ production of ROS via fluorescence microscopy.25) Frozen tissue sections of the myocardium (ca. 10 µm) were incubated with DHE (8 µM) at 37°C for 1 h. Images were taken using a Spot RT digital camera (Diagnostic Instruments), which was connected to a fluorescence microscope (Nikon Eclipse 80i; Nikon, Tokyo, Japan). The excitation and emission wavelengths were 490 and 530 nm, respectively. The fluorescence signal densities were analyzed using ImageJ software (NIH, Bethesda, MD, U.S.A.). The levels of 3-nitrotyrosine (3-NT) and cGMP in myocardial tissue were determined using a 3-NT and cGMP enzyme-linked immunosorbent assay (ELISA) kit (Shanghai Enzyme-linked Biotechnology Co., Ltd., Shanghai, China). After the heart was removed, three hearts were preserved according to the experimental method, and five different sites of each heart were used for the determination of ROS and detection of cGMP. The level of NO in myocardial tissue was determined using a NO assay kit (Nanjing Jiancheng Bioengineering Institute, Jiangsu, China). The concentrations of nitrate and nitrite were considered indicators for NO production in myocardial tissue and determined by a double antibody sandwich method according to the instructions of the reagent kit.
Determination of Cardiac Biopterins and eNOS ActivityCardiac biopterins were measured as previously reported.26,27) Hearts were rapidly excised and stored at −80°C. A Waters-2695 HPLC (Waters Corporation, Milford, MA, U.S.A.) with a differential oxidation method described previously was used to measure BH4 and BH2 concentrations (Waters 2475 multi-wavelength fluorescence detector, Waters Corporation) in homogenized heart samples (n = 5).
NOS activity was examined by measuring the conversion of L-arginine to citrulline, according to the instructions of the kit manufacturer (PerkinElmer, Inc., Waltham, MA, U.S.A.). The filtrate was quantified by liquid scintillation counting.
Reverse Transcription and Quantitative Real-Time PCR (qRT-PCR)Total RNA was extracted from cells by using Trizol reagent (Invitrogen, Carlsbad, CA, U.S.A.) after treatment. Reverse transcription of total RNA (1 µg) was performed using a TaKaRa reverse transcription kit (TaKaRa Biotechnology, Dalian, China). The expression of individual genes was then analyzed by semi-quantitative qRT-PCR using SYBR green technology (TaKaRa). The primers for rat eNOS: forward primer (F): 5′-GTG CTG GCA TAC AGA ACC CA-3′, reverse primer (R): 5′-CCT GCC TTG AGT TGG CTC AT-3′; sarcoplasmic reticulum Ca2+ ATPase (SERCA2a), F: 5′-GTT CTG CTG CAC AGT AGG GAT-3′, R: 5′-AGG CCA GCA GAA ACT TGA GTA A-3′. Phospholamban (PLN), F: 5′-TGG TGA ATG GTC TGC GGA AT-3′, R: 5′-CAG GCG CTT TTC ACC TTC CT-3′ and actin cytoplasmic 1 beta (ACTB), F: 5′-AGT ACA ACC TTC TTG CAG CTC CTC-3′; R: 5′-TGC CGG AGC CGT TGT CG-3′. PCR was performed for 40 cycles with the following parameters: 3 min at 95°C, and for each cycle 30 s at 95°C for denaturation and 20 s at 55°C for annealing. All quantitative RT-PCR analyses were conducted using a 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, U.S.A.). The relative mRNA expression of PLN, SERCA2a, and eNOS was calculated using the 2−ΔΔCT method and normalized to that of ACTB mRNA.
Western Blot AnalysisEqual amounts of myocardial protein (40 µg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride membranes. The blots were incubated with the primary antibodies for β3-adrenoceptor (Sigma, St. Louis, MO, U.S.A.), eNOS (CST, Danvers, MA, U.S.A.), SERCA2a (Abcam, Cambridge, MA, U.S.A.), Phosphorylated PLN (phospho S16, Abcam), PKG (ProteinTech Group, Chicago, IL, U.S.A.) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Abcam), respectively. All the protein bands were scanned, and relative integrated density values (IDVs) were calculated by Quantity One software (Bio-Rad Laboratories, Hercules, CA, U.S.A.) and normalized to that of GAPDH (n = 5).
Immunohistochemical StainingHearts were arrested in diastole using pressure perfusion of 10% KCl. The frozen samples were sectioned at a thickness of 6 mm from mid-LV level and fixed in 10% formalin. A total of 15 tissue sections from 3 hearts in each group were incubated with primary antibodies for β3-adrenoceptor and cGMP (Zhenjiang Hope Biotechnology Co., Ltd., Jiangsu, China) overnight at 4°C and incubated with the secondary antibodies for 1 h at 37°C. The sections were then stained with diaminobenzadine. Quantitative protein expression values were calculated by density mean value using Image-Pro Plus 7.0 analysis software.
Statistical AnalysisSPSS 19.0 software (SPSS, Inc., Chicago, IL, U.S.A.) was used for statistical analysis. Data are presented as mean ± standard deviation (S.D.). Data for SHR baseline and WKY were compared with a two-tailed Student t-test. One-way ANOVA was used to compare multiple SHR groups, and post hoc multiple comparisons (least significant difference test) were also conducted. Two-way ANOVA was used to investigate the interaction between the effects of BH4 and nebivolol. Covariance analysis was used to assess the difference between the outcomes of a non-invasive index after treatment. p < 0.05 was considered statistically significant.
SBP and heart rate were significantly elevated in baseline SHRs compared with those in age-matched WKY rats (Table 1). The value of E′/A′ in SHRs was significantly lower than that in WKY rats (Fig. 1A). The E/E′ ratio of SHRs was significantly increased compared with that in WKY rats, which is consistent with impaired diastolic function. The results of invasive hemodynamic evaluation were also in accordance with the echocardiographic data (Table 1). Compared with WKY rats, SHRs had prolonged time constants for isovolumic relaxation (tau, p < 0.01, Table 1). Furthermore, baseline SHRs had a significantly greater LV end-diastolic pressure (LVEDP) and thicker LVPWd than those in WKY rats (p < 0.01, p = 0.012, respectively). These data suggested that SHRs had diastolic dysfunction of the LV. Moreover, we also measured the baseline characteristics of cardiac function in all groups before treatment and found no differences among the SHR-originated groups (p > 0.05, Supplementary Table 1).
| WKY (n = 8) | SHR (n = 8) | p | |
|---|---|---|---|
| Body weight (g) | 251.03 ± 12.89 | 249.86 ± 5.05 | 0.81 |
| Noninvasive measures | |||
| SBP (mmHg) | 124.16 ± 6.72 | 207.66 ± 10.11 | <0.001 |
| HR (bpm) | 363.52 ± 10.7 | 426.23.00 ± 7.51 | 0.001 |
| Diastolic measures | |||
| E (mm/s) | 664.21 ± 6.98 | 648.32 ± 11.51 | 0.10 |
| A (mm/s) | 265 ± 14.01 | 292 ± 14.85 | 0.014 |
| E′ | 45.12 ± 1.55 | 27.37 ± 1.84 | <0.001 |
| A′ | 29.49 ± 1.77 | 32.58 ± 1.83 | 0.01 |
| E/A | 2.50 ± 0.15 | 2.23 ± 0.13 | 0.012 |
| E/E′ | 14.73 ± 0.50 | 23.93 ± 0.83 | 0.001 |
| E′/A′ | 1.53 ± 0.12 | 0.84 ± 0.05 | <0.001 |
| Systolic measures | |||
| FS (%) | 40.42 ± 0.2 | 41.40 ± 0.21 | NS |
| EF (%) | 74.60 ± 0.41 | 74.72 ± 1.12 | NS |
| LV dimensions | |||
| LVPWd (mm) | 1.25 ± 0.05 | 1.35 ± 0.09 | 0.012 |
| LVEDd (mm) | 6.02 ± 0.05 | 6.03 ± 0.06 | NS |
| LVESd (mm) | 3.44 ± 0.07 | 3.52 ± 0.10 | 0.115 |
| Invasive measures | |||
| HR (bpm) | 348.87 ± 48.0 | 382.77 ± 47.03 | 0.012 |
| Systolic function | |||
| LVSP | 102.57 ± 4.83 | 153.68 ± 11.53 | <0.001 |
| dP/dtmax (mmHg/s) | 8023 ± 122.00 | 8234 ± 86.77 | 0.11 |
| Diastolic function | |||
| LVEDP | 10.60 ± 4.04 | 21.25 ± 1.01 | <0.001 |
| dP/dtmin (mmHg/s) | −5658 ± 149.51 | −6334 ± 128.32 | <0.001 |
| dP/dtmin/LVSP (s−) | −55.21 ± 1.82 | −41.94 ± 5.93 | 0.004 |
| Tau | 15.81 ± 1.00 | 30.34 ± 1.52 | <0.001 |
WKY, Wistar-Kyoto rats; SHR, spontaneously hypertensive rats; SBP, systolic blood pressure; HR, heart rate; E = peak early diastolic LV filling velocity; A = peak late diastolic LV filling velocity; E/A = peak E and A velocity ratio; E′ and A′, maximal velocity of mitral annulus during early and late diastole; FS, fractional shortening; LVEF, LV ejection fraction; LVPWd, LV posterior wall thickness in diastole; LVEDd, LV end-diastolic dimension; LVESd, LV end-systolic dimension; LVSP, LV systolic pressure; LVEDP, LV end-diastolic pressure; tau, time constant of isovolumic LV relaxation; ±dP/dtmax: maximal rate of pressure rise/decline. Values are mean ± S.D.

(A) Evaluation of LV function in WKY rats and SHRs. Left: A greater LVPWd was found in SHRs compared to WKY rats by M-mode echocardiographic recordings. Middle: Transmitral flow Doppler showed lower E/A in SHRs compared to WKY rats. Right: Mitral annulus tissue Doppler showed lower E′/A′ in SHRs compared to WKY rats. (B) Evaluation of LV function in different SHR groups. Left: Examples of transmitral flow Doppler, showing E and A waves in four groups. Right: Example of mitral annulus tissue Doppler, showing different degrees of benefit in treated groups, especially in the N&BH4, N, BH4 groups with an E′/A′ >1.
Treatment with BH4 effectively improved diastolic function in SHRs. The invasive and noninvasive diastolic indexes (including LVEDP, −dP/dtmin, −dP/dtmin/LVSP, tau, E/E′ and E′/A′) were improved in the BH4 group compared with those in the control group (all p < 0.01, Table 2, Fig. 1B). Similarly, treatment with nebivolol promoted the hemodynamic status and LV function in SHRs. Diastolic indexes including LVEDP, −dP/dtmin/LVSP, tau, E/E′ and E′/A′ were significantly improved in the N group compared with those in the control group (all p < 0.01, Table 2, Fig. 1B).
| Control (n = 8) | BH4 (n = 8) | N (n = 8) | N&BH4 (n = 8) | |
|---|---|---|---|---|
| Body weight (g) | 259.80 ± 5.01 | 258.62 ± 5.22 | 258.11 ± 5.72 | 260.15 ± 4.72 |
| Noninvasive measures | ||||
| SBP (mmHg) | 205.75 ± 2.06 | 201.17 ± 3.27 | 199.40 ± 1.14** | 198.58 ± 1.65** |
| HR (bpm) | 422.33 ± 6.59 | 417.16 ± 5.67## | 362.16 ± 17.33** | 358.15 ± 5.05** |
| Diastolic measures | ||||
| E (mm/s) | 648.16 ± 4.26 | 654.00 ± 7.24 | 647.50 ± 6.28## | 666.00 ± 5.25** |
| A (mm/s) | 297.33 ± 6.36 | 286.66 ± 6.58**,## | 289.83 ± 4.16*,## | 273.20 ± 4.43** |
| E/A | 2.18 ± 0.32 | 2.28 ± 0.69**,## | 2.23 ± 0.38**,## | 2.41 ± 0.59** |
| E′ | 26.75 ± 1.66 | 34.36 ± 2.5**,## | 35.81 ± 1.35**,## | 38.26 ± 1.16** |
| A′ | 32.25 ± 2.12 | 32.75 ± 1.66## | 33.24 ± 1.75## | 27.50 ± 1.60** |
| E/E′ | 24.15 ± 0.72 | 19.12 ± 1.34**,## | 18.08 ± 0.81**,# | 17.02 ± 0.54** |
| E′/A′ | 0.83 ± 0.02 | 1.05 ± 0.06**,## | 1.08 ± 0.02**,## | 1.39 ± 0.38** |
| Invasive measures | ||||
| HR (bpm) | 389.2 ± 34.6 | 388.4 ± 38.9 | 378.5 ± 19.4* | 372.72 ± 41.5* |
| Systolic function | ||||
| LVSP | 154.88 ± 0.77 | 151.51 ± 1.28## | 147.59 ± 1.26** | 146.56 ± 1.57** |
| LVEF | 74.39 ± 0.46 | 74.62 ± 0.43 | 75.20 ± 0.53 | 74.82 ± 0.34 |
| LVFS | 40.12 ± 0.54 | 40.32 ± 0.34 | 40.71 ± 0.54 | 41.02 ± 0.22 |
| +dP/dtmax (mmHg/s) | 8337 ± 167.23 | 8257 ± 63.35 | 8224 ± 146.34 | 8228.8 ± 66.56 |
| Diastolic function | ||||
| LVEDP | 21.44 ± 1.33 | 16.64 ± 0.91**,# | 16.24 ± 0.41**,# | 13.50 ± 0.71** |
| −dP/dtmin (mmHg/s) | −6366 ± 57.42 | −6868 ± 54.35**,## | −6684 ± 95.46**,## | −7202 ± 93.21** |
| −dP/dtmin/LVSP (s−) | −41.33 ± 1.6 | −45.48 ± 0.94**,## | −46.46 ± 1.82**,## | −49.33 ± 1.15** |
| Tau | 31.24 ± 0.61 | 25.16 ± 0.98**,## | 23.32 ± 0.94**,## | 21.07 ± 0.62** |
Values are mean ± S.D. SBP, systolic blood pressure; HR, heart rate; E = peak early diastolic LV filling velocity; A = peak late diastolic LV filling velocity; E/A = peak E and A velocity ratio; E′ and A′, maximal velocity of mitral annulus during early and late diastole; LVFS, LV fractional shortening; LVEF, LV ejection fraction; LVPWd, LV posterior wall thickness in diastole; LVEDd, LV end-diastolic dimension; LVSP, LV systolic pressure; LVEDP, LV end-diastolic pressure; tau, time constant of isovolumic LV relaxation; ±dP/dtmax: maximal rate of pressure rise/decline. * p < 0.05, ** p < 0.01 compared with the control group (SHR placebo); # p < 0.05, ## p < 0.01 compared with the N&BH4 group.
The cardiac diastolic function of SHRs was evaluated after treatment with BH4 combined with nebivolol. The noninvasive SBP and heart rate were both significantly decreased in combined treatment group compared with those in the control group (p < 0.05 or p < 0.01, Table 2). However, there was no significant difference in the noninvasive SBP and heart rate between the N&BH4 and N treatment groups (p > 0.05, Table 2). The invasive and noninvasive diastolic indexes (including tau, LVEDP, −dP/dtmin/LVSP, E/E′ and E′/A′) were improved in the combined treatment group compared with those in the control group (p < 0.01). The E/E′, LVEDP, and tau were significantly lower in the N&BH4 group than those in the BH4 and nebivolol groups (p < 0.05 or p < 0.01, Table 2). The LVEDP in the N&BH4 group was significantly lower than that in the N group (p < 0.01, Table 2). Also, the E′/A′ and dP/dtmin/LVSP were significantly greater in the N&BH4 group than those in BH4 or N group (p < 0.05 or p < 0.01, Table 2). These data suggested that BH4 and nebivolol synergistically improved cardiac diastolic function in SHRs.
Two-way ANOVA indicated no significant interaction between the effects of BH4 and nebivolol on SBP, heart rate, eNOS, and LVSP (all p > 0.05). However, there was a significant interaction between the effects of BH4 and nebivolol on noninvasive indexes (including E/E′ and E′/A′) and invasive indexes (including tau, LVEDP and −dP/dtmin/LVSP; all p < 0.05).
Combined Use of BH4 and Nebivolol Synergistically Down-Regulated the Level of Cardiac Oxidative Stress in MyocardiumDHE, in situ marker of ROS production in myocardium, can be rapidly oxidized to ethidium bromide in the presence of superoxide. The fluorescence intensity of DHE was significantly decreased after treatment with BH4 or nebivolol compared with that in the control group (both p < 0.01). In addition, the N&BH4 group had a significantly lower fluorescence intensity for DHE than that in the monotherapy treatment groups (all p < 0.01, Figs. 2A, B). 3-NT is another in vivo marker of oxidative stress, particularly caused by ONOOˉ, a reaction product of NO and superoxide (O2−). The level of 3-NT was significantly decreased after treatment with BH4 or nebivolol compared with that in the control group (both p < 0.01, Fig. 2C). Interestingly, the N&BH4 group had an even significantly lower 3-NT level than those in monotherapy treatment groups (p < 0.01 or p < 0.05, Fig. 2C). Two-way ANOVA indicated a significant interaction between the effects of BH4 and nebivolol on down-regulating ROS in myocardium (p < 0.05).
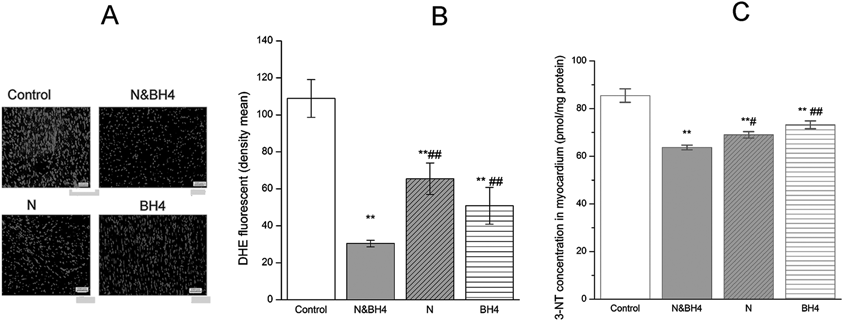
Control group: treated with saline (0.9%); N&BH4 group: treated with nebivolol (5 mg/kg/d) + BH4 (25 mg/kg/d); N group: treated with nebivolol (5 mg/kg/d); BH4 group: treated with BH4 (25 mg/kg/d). (A) Fluorescent photomicrographs of in situ labeling of reactive oxygen species (ROS, 4 × 10) production in myocardium. (B) Summary data of myocardium section in each group, measured by DHE emission at 530 nm when excited at 490 nm (n = 15). (C) 3-NT concentration in myocardium by ELISA (n = 5). ** p < 0.01 compared with the control group; # p < 0.05, ## p < 0.01 compared with the N&BH4 group.
BH4 is an essential cofactor for NOS, which can become uncoupled when BH4 is oxidized. BH4, nebivolol or a combination treatment significantly up-regulated the mRNA and protein expression of eNOS in the myocardium compared with those in the control group (Fig. 3A, Supplementary Fig. 2). In particular, the N&BH4 group showed the most significantly increased expression level of eNOS dimer (p < 0.01 or p < 0.05, Fig. 3B, Supplementary Fig. 1). Although our data showed that BH4 rather than nebivolol significantly increased BH4 content in myocardium, BH4, nebivolol or a combination of them elevated the ratio of BH4 to BH2 and also improved eNOS activity (all p < 0.01, Figs. 3C, 4A–C). Furthermore, the N&BH4 group had a higher BH4/BH2 ratio and eNOS activity than those in the monotherapy groups (p < 0.05 or p < 0.01 Figs. 3C, 4C). NO production was significantly enhanced in the myocardium of the N, BH4 and N&BH4 groups compared with that in the control group. Moreover, N&BH4 exhibited a better effect than either monotherapy (p < 0.05 or p < 0.01, Fig. 3D). Two-way ANOVA indicated a significant interaction between the effects of BH4 and nebivolol on BH4/BH2, eNOS activity, and NO production (all p < 0.05).
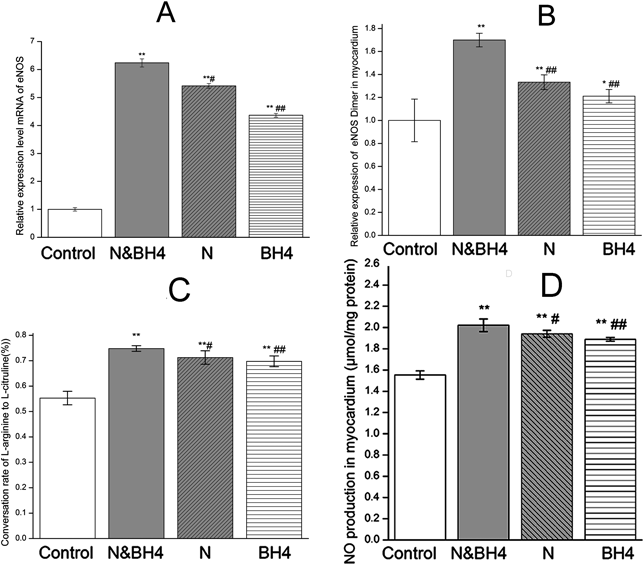
Control group: treated with saline (0.9%); N&BH4 group: treated with nebivolol (5 mg/kg/d) + BH4 (25 mg/kg/d); N group: treated with nebivolol (5 mg/kg/d); BH4 group: treated with BH4 (25 mg/kg/d). (A) The effect of BH4 and/or nebivolol on the mRNA expression of eNOS. eNOS expression in each group was normalized to β-actin expression (n = 5). (B) The relative protein expression of eNOS dimer in myocardium. (C) Activity of eNOS, as measured by the ratio of [14C]-labeled arginine to citrulline (n = 5). (D) NO production in myocardial tissue, as determined using a NO assay kit (n = 5). * p < 0.05, ** p < 0.01 compared with the control group; # p < 0.05, ## p < 0.01 compared with the N&BH4 group.

(A) Representative concentrations of BH4 and BH2 in the myocardium as determined by HPLC. The concentrations of BH4 and BH2 were calculated by single point correction according to the fluorescence intensity (ordinate) and peak time (abscissa) (n = 5). (B) BH4 content in myocardium (n = 5). (C) Ratio of BH4/BH2 (n = 5 in each group). ** p < 0.01 compared with the control group; ## p < 0.01 compared with the N&BH4 group.
We investigated cGMP and β3-adrenoceptor expression in the myocardium via immunohistochemical staining. The levels of cGMP or β3-adrenoceptor expression were significantly increased in the combined treatment group as well as the monotherapy groups compared with that in the control group (p < 0.05 or p < 0.01, Figs. 5A, B, Supplementary Fig. 3). In addition, the level of cGMP or β3-adrenoceptor expression in the N&BH4 group was significantly higher than those in the BH4 and N groups (p < 0.01, p < 0.05, respectively, Figs. 5A, B, Supplementary Fig. 3). The levels of cGMP and β3-adrenoceptor expression in the myocardium were significantly up-regulated in all of the treatment groups compared with those in the control group (all p < 0.01, Figs. 5C, D).
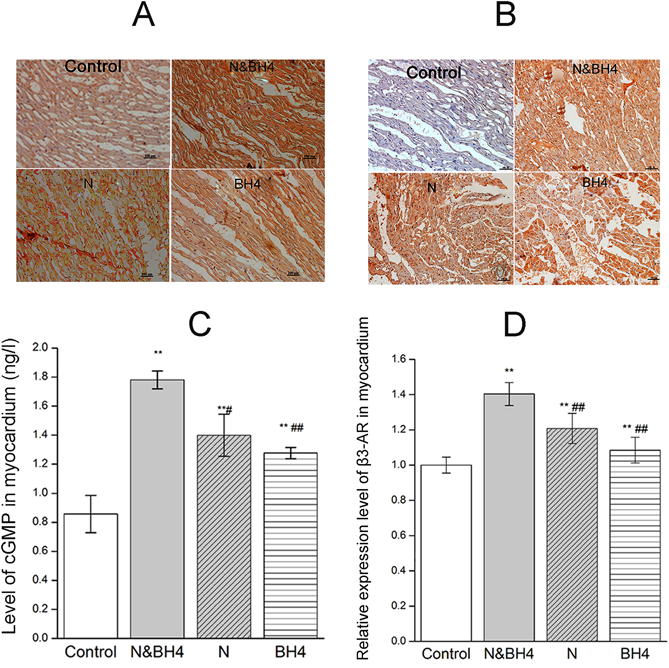
Control group: treated with saline (0.9%); N&BH4 group: treated with nebivolol (5 mg/kg/d) + BH4 (25 mg/kg/d); N group: treated with nebivolol (5 mg/kg/d); BH4 group: treated with BH4 (25 mg/kg/d). Representative concentrations cGMP (A) and β3-adrenoceptor (B) in myocardium were determined by immunohistochemical analysis. Brown granules of cGMP were expressed mainly in the cytoplasm of the myocardium, and β3-adrenoceptor was expressed mainly in the cytomembrane of the myocardium. The expression levels of cGMP and β3-adrenoceptor in the N&BH4 group were higher than those in the N, BH4 or control group (n = 15). The bottom right scale bar (—) is 100 µm. (C) cGMP content, as determined by ELISA (n = 5). (D) β3-adrenoceptor expression in the myocardium, as determined by Western blotting (n = 5). ** p < 0.01 compared with the control group; # p < 0.05, ## p < 0.01 compared with the N&BH4 group. (Color figure can be accessed in the online version.)
The expression of PKG was higher in all of the treatment groups compared with that in the control group, and PKG expression was significantly elevated in the N&BH4 group compared with the monotherapy groups (p < 0.05 and p < 0.01, respectively, Fig. 6A). BH4 and/or nebivolol treatment increased the expression of PLN mRNA, SERCA2a mRNA and protein, and phosphorylated PLN (pPLN) in the myocardium compared with levels in the control group (p < 0.01 or p < 0.05, Figs. 6B–E, Supplementary Fig. 1). Although the expression level of SERCA2a protein in the N&BH4 group was lower than that in BH4 group, the ratio of pPLN to SERCA2a was greater than those in monotherapy treatment groups (p < 0.01, Fig. 6F). Two-way ANOVA indicated no significant interaction between the effects of BH4 and nebivolol on β3-adrenoceptor expression (p > 0.05), but significant interactions with the cGMP level and the protein expression of PKG, pPLN/SERCA2a, and eNOS dimer (all p < 0.05). These data suggested that the effects of BH4 and/or nebivolol on the myocardium may be mediated by the NO/cGMP/PKG signaling pathway.

Control group: treated with saline (0.9%); N&BH4 group: treated with nebivolol (5 mg/kg/d) + BH4 (25 mg/kg/d); N group: treated with nebivolol (5 mg/kg/d); BH4 group: treated with BH4 (25 mg/kg/d). Relative protein expression of PKG (A), pPLN (D), SERCA2a (E) ratio of SERCA2a/PLN (F) in myocardium. Protein expression levels were normalized to GAPDH expression determined by Western blot analysis (n = 5 in each group). mRNA expression levels of PLN (B) and SERCA2a (C) in myocardium. Expression levels in each group were normalized to β-actin expression (n = 5). ** p < 0.01 compared with the control group; # p < 0.05, ## p < 0.01 compared with the N&BH4 group.
In hypertension, oxidative stress in the myocardium results from eNOS uncoupling and reduced NO bioavailability and leads to diastolic dysfunction.3,28,29) In the present study, monotherapy or combined use of BH4 and nebivolol increased the expression levels of eNOS dimer and total eNOS (Supplementary Fig. 2) and elevated the activity of eNOS and NO production. This effect is not only correlated with the BH4 level in the myocardium, but more importantly, with the BH4/BH2 ratio. It is worth mentioning that both BH4 and nebivolol reduced the level of the fluorescence intensity of DHE and 3-NT, markers of oxidative stress in the myocardium.25,30) These data suggest that BH4 and nebivolol have cardioprotective effects through their antioxidant capability, supporting the recoupling of eNOS and augmenting NO production. This finding is in line with our previous study.31) In hypertensive patients, nebivolol also has better renal protective effects, including better preservation of the glomerular filtration rate and renal blood flow compared with atenolol, another β1-adrenoceptor antagonist.32)
It is well known that cGMP dependent protein kinase (PKG) represents a downstream signal of NO synthase. Our data showed that BH4 and/or nebivolol not only enhanced the NO production by improving eNOS dimer, but also increased its activity due to its recoupling. cGMP content and protein expression and PKG expression were both found increased during this process. Abdallah et al.33) demonstrated that cGMP signaling corresponded to increased storage of Ca2+ in the endoplasmic reticulum, resulting from improved SERCA2a activity. However, SERCA activity depends on phosphorylation of its regulating protein, PLN.34) An inhibitor of PKG can decrease the phosphorylation of PLN.33) In heart, SERCA2a activity controls both the degree of SR Ca2+ loading and the rate of cytosolic Ca2+ removal, which is closely related to cardiac relaxation and contraction.35) The present study indicated that BH4 and/or nebivolol increased the expression of pPLN and the ratio pPLN to SERCA2a in myocardium. This ratio was especially higher in the N&BH4 treatment group than in other treatment groups.
We propose that BH4 and nebivolol have a synergistic protective effect in heart failure. In this study, the N&BH4 group displayed significant improvement in noninvasive and invasive measures compared to the BH4 or N group. The N&BH4 group had a more down-regulated intensity of DHE fluorescence and decreased 3-NT level than the monotherapy groups. The expression of eNOS dimer, β3-adrenoceptor, eNOS activity, tissue nitrate and nitrite, and the noninvasive and invasive measures of diastolic function, are linked by established cause-and-effect relationships. Moreover, N&BH4 treatment showed a stronger effect due to the corresponding higher expression levels of β3-adrenoceptor, eNOS dimer, and BH4/BH2 as well as the production of eNOS-derived NO compared to the BH4 or N group. The expression of cGMP, the effector of the NO pathway, was significantly enhanced in the N&BH4 group compared with those in the control, BH4, and N groups. The primary downstream target of vascular smooth muscle cGMP is PKG, which reduces titin-based stiffness to improve diastolic function in human hearts,36) was significantly elevated in the N&BH4 group compared with the monotherapy groups. These effects of combined use of BH4 and nebivolol are consistent with the reduction in blood pressure in the N&BH4 group. These data suggest that the combination therapy is more effective at preventing cardiac dysfunction than monotherapy with BH4 or nebivolol, and multiple signaling molecules and effectors are involved in the complicated regulatory networks.
Fang et al.20) confirmed that nebivolol treatment improved left ventricular diastolic function via enhancement of NO bioavailability and was completely abolished by the NO synthase inhibitor Nω-Nitro-L-arginine (L-NNA). Zhang et al.37) also reported the cardio-protective effects of nebivolol could be abolished by the β3-adrenoceptor antagonist SR59230A. Barr et al.38) reported that due to increases in superoxide levels through eNOS uncoupling, exercise could increase the size of the myocardial infarction area in β3-adrenoceptor knockout mice, and treatment with BH4 significantly reduced this myocardial injury. Specifically, β3-adrenoceptor-deficient mice showed exacerbated remodeling in pressure-induced heart failure,39) and mice with overexpression of β3-adrenoceptor displayed diminished neurohormone-induced hypertrophic remodeling.40) These findings and our data suggest that nevibolol can decrease oxidative stress, supporting the recoupling of eNOS and augmenting NO production by activating the β3-adrenoceptor. There were no significant differences in the SBP and heart rate between the N&BH4 and N groups. This indirectly indicates that N&BH4 may play a synergistic effect independently of the antihypertensive effect of nebivolol and this effect is closely associated with the NO pathway.
According to our data, we propose the following scheme as an explanation for BH4 and nebivolol-mediated protective effects against diastolic dysfunction. (i) BH4 and/or nebivolol can maintain relative higher BH4/BH2 via antioxidation and decrease the oxidative stress in myocardium of hypertension. (ii) Nebivolol not only activates eNOS activity via β3-adrenoceptor pathway but also increases eNOS activity with BH4 via recoupling eNOS. (iii) Activation of eNOS leads to an increased level of cGMP and activation of PKG, which increases the phosphorylation of PLN and consecutively activates SERCA2a. (iv) Activated SERCA allows Ca2+ to sequestrate into the SR so as to improve the diastolic ability.
There are some limitations in this study. Although we evaluated β3-adrenoceptor expression in the myocardium by immunohistochemical staining and the results provided some evidence concerning the relationship between β3-adrenoceptor and eNOS, the activity of the compound via the β3-adrenoceptor pathway requires further investigation. The present study did not include an investigation of the expression of β-adrenoceptor subtypes, and the detailed pathological and physiological functions of β3-adrenoceptor have not been extensively investigated. Moreover, there are still intense debates concerning which NOS isoforms plays a crucial role in β3-adrenoceptor signaling. Our findings suggest that N&BH4 treatment is beneficial for improving diastolic dysfunction and could be used as a new therapeutic strategy. Further studies are needed to determine the exact mechanism.
We confirm that the administration of BH4 or nebivolol has a protective effect on diastolic function in SHRs. BH4 combined with nebivolol can exert a synergistically cardioprotective effect through activation of β3-adrenoceptor and the NO/cGMP/PKG signaling pathway.
This work was supported by the National Natural Science Foundation of China (No. 81270332), Gansu Province Natural Science Foundation (110FKCA150 and 145RJZA174) and Gansu Province Health Industry Scientific Research Plan (GSWSKY-2014-33).
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.