2019 年 42 巻 8 号 p. 1423-1427
2019 年 42 巻 8 号 p. 1423-1427
Age is known as one of influencing factor for theophylline (TP)-metabolizing capacity. In a previous our study, the ratio of TP and its major metabolite 1,3-dimethyluric acid (DMU) in serum (DMU/TP) is a useful index to estimate TP-metabolizing capacity, and this value markedly increased by influencing factor, such as the history of smoking. However, it is unknown whether DMU/TP values in serum reflect age-associated changes of TP-metabolizing capacity. In this study, the effect of age on the DMU/TP values in serum were investigated using mice of different age due to the limited blood sampling in human. The concentrations of TP and its metabolites in mouse serum were simultaneously measured using HPLC. As observed in human serum, serum TP concentrations were closely correlated with DMU concentration in mice, which indicates that the DMU/TP ratio is a good indicator of TP metabolic ability in mice. When TP was administered subcutaneously in 2–28-week-old mice, age-associated changes in the DMU/TP ratio in mice were observed. In conclusion, age-associated changes in TP-metabolizing capacity can be estimated by the DMU/TP ratio in serum.
Theophylline (TP), including aminophylline, is a xanthine derivative and is used worldwide to treat bronchial diseases such as acute and chronic asthma and chronic obstructive pulmonary disease.1,2) The therapeutic range of TP is narrow (10–20 µg/mL), and adverse effects occur above plasma concentrations of 20 µg/mL.3) Thus, patients taking TP are recommended to individually monitor TP levels in serum for optimal dosing, which is referred to as therapeutic drug monitoring.
The metabolic pathways of TP are as follows: the main metabolic pathway is hydroxylation at position 8, which converts TP to 1,3-dimethyluric acid (DMU), and another metabolic pathway is N-demethylation, which converts TP into either 3-methylxanthine (3MX) or 1-methylxanthine (1MX). Furthermore, 1MX is rapidly metabolized into a final metabolite 1-methyluric acid (1MU) by xanthine oxidase.4) Serum TP concentration is closely correlated with DMU concentration in patients with asthma or bronchial asthma; the concentration ratio (DMU/TP) in patients is fixed with a normal ability to metabolize TP, but this value changes markedly in patients with a history of smoking.5–7) Therefore, the TP-metabolizing capacity of each patient can be estimated by the ratio of TP and DMU (DMU/TP) in serum, which can be rapidly and simultaneously measured using HPLC. In addition to smoking, some biological and external factors affect TP pharmacokinetics, especially metabolism.8) For example, age is an important covariate for TP pharmacokinetics.8,9) Thus, the DMU/TP value may reflect changes in TP-metabolizing capacity when patients become order. However, it is unknown whether the serum levels of DMU/TP after TP administration reflect changes in TP-metabolizing capacity with respect to age.
Here, we aimed to investigate the effects of age on the serum levels of DMU/TP after TP administration to estimate TP-metabolizing capacity. However, it is difficult to sample blood serially in people and evaluate changes in TP serum levels and metabolites. To overcome this issue, we conducted the study using mice instead of human. To achieve this, we first established a method to determine the levels of TP and its metabolites in serum using HPLC in mice. We then investigated a significance of the DMU/TP for predicting the TP-metabolizing capacity in mice. Finally, we determined DMU and TP serum levels in mice of different ages.
TP, DMU, 1MU, 1MX, 3MX, and β-hydroxyethyltheophylline were purchased from Sigma-Aldrich (Tokyo, Japan). Acetonitrile (HPLC grade) and methanol (HPLC grade) were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). All other chemicals and reagents were of the highest grade commercially available unless otherwise noted, and all solutions were prepared using deionized water.
AnimalsMale ICR mice were purchased from Sankyo Labo Service (Tokyo, Japan) and given food and water ad libitum in a temperature-controlled room with a 12-h dark/light cycle. Mice were housed in a conventional room for at least 1 week before use in experiments. The animals were handled according to the “Guiding Principles for the Care and Use of Laboratory Animals of the Japanese Pharmacological Society” and “Rules Concerning Animal Experiments, etc., Keio University Faculty of Pharmacy.” All animal experiments were reviewed and approved by the Animal Care and Use Committee of Keio University.
Blood Samples CollectionAll mice were subcutaneously administered TP at a dose of 45 mg/kg. This dose is within dose-proportional. In addition, all of the mice survived after the administration of TP and no side effect symptom was observed. Blood was sampled by heart puncture under anesthesia at pre-determined times (3- and 7-week-old mice: 10, 20, 30, 60, 120, and 180 min: 2-, 5-, 7-, 12-, 20-, and 28-week-old mice: 60 min). Blood samples were left for 30 min at room temperature and then centrifuged to collect serum (3000 rpm, 15 min). Serum samples were stored by freezing at −80°C until use.
Quantification of TP and Its Metabolites by HPLCThe serum concentrations of TP and its metabolites (DMU, 1MU, 1MX, and 3MX) were simultaneously determined as described previously.6) Briefly, serum samples (100 µL) were deproteinized by addition of 300 µL acetonitrile solution containing 0.3 µg β-hydroxyethyltheophylline as an internal standard and centrifuged (14000 rpm, 5 min). An aliquot of supernatant (300 µL) was evaporated, and all of the dried samples were reconstituted in 60 µL of the mobile phase. TP and its metabolites were analyzed using HPLC interfaced with a TSK gel ODS-80TM (E3115, 250 × 4.6 mm i.d., Tosoh Co., Tokyo, Japan), an L-7420 UV detector (Hitachi Ltd., Tokyo, Japan), an L-7100 pump (Hitachi Ltd.), and an L-7200 autosampler (Hitachi Ltd.). The mobile phase was composed of 20 mM sodium acetate buffer (pH 4.8), acetonitrile, and methanol (90 : 2 : 8 (v/v)) with a flow rate of 1 mL/min at 40°C. The UV detector wavelength was fixed at 275 nm. Serum TP and metabolite levels were calculated from the peak area ratio relative to that of the internal standard using a calibration curve.
StatisticsAll data are expressed as the mean ± standard deviation (S.D.). TP pharmacokinetic parameters were analyzed using the Phoenix® WinNonlin® software program (Version 8.0; Certara LP, Princeton, NJ, U.S.A.). Statistical analyses for multiple comparisons were determined by the ANOVA (two-way ANOVA). A Spearman test was used for correlation analyses. A probability value of p < 0.05 was considered significant.
We previously established an HPLC method to simultaneously quantify TP and its metabolites in human serum6) and used this method to measure TP and its metabolites in mouse serum. Figure 1 shows a representative HPLC chromatogram of serum from a mouse administered TP. In mouse serum, no interfering peak was detected, and the TP and its metabolites (1MU, 3MX, and DMU) were clearly separated within 30 min, which is similar to the HPLC chromatogram of human serum (Fig. S1). However, 1MX, which is an intermediate metabolite from TP to 1MU, was not detected in mouse serum because 1MX is rapidly metabolized into 1MU by xanthine oxidase and excreted in urine.10) Thus, the method for the simultaneous measurement of TP and its metabolites in mouse serum was established based on the method in humans.
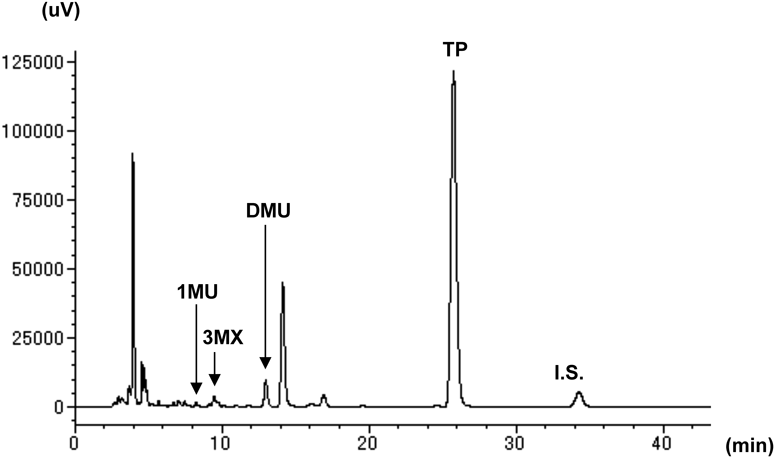
1MU, 1-methyluric acid; 3MX, 3-methylxanthine; DMU, 1,3-dimethyluric acid; TP, theophylline; IS, β-hydroxyethyltheophylline.
To evaluate whether the serum level of DMU/TP reflected the TP-metabolizing ability in mice, changes in the serum levels of TP and its metabolites were determined after subcutaneous administration of TP. Figure 2 shows the time-course serum concentration of TP and its metabolites in 3-week-old and 7-week-old mice, and the corresponding pharmacokinetic parameters of TP were calculated using a non-compartmental analysis. The amounts of TP metabolites were in the order DMU > 1MU > 3MX in both 3-week-old and 7-week-old mice (Fig. 2), which indicates that DMU is the major metabolite of TP in ICR mice similar to that in other kinds of mice and in humans.11–13) In addition, pharmacokinetic parameters of TP were as follows in 3-week-old and 7-week-old mice: an elimination rate constant (ke) of 0.658 and 0.859 h−1, clearance (CL/F) of 7.32 and 11.6 mL/h, peak plasma concentration (Cmax) of 54.9 and 71.8 µg/mL, and peak plasma concentration (Tmax) of 0.17 and 0.33 h, respectively.

All ICR mice were subcutaneously administered TP at a dose of 45 mg/kg, and blood was sampled 10, 20, 30, 60, 120, and 180 min after TP administration. The values are the mean ± S.D. (n = 5–6).
Previously, we found that serum TP concentrations were closely correlated with the DMU concentration in patients with asthma or bronchial asthma, which results in an almost constant DMU/TP ratio.5,6) Therefore, the DMU/TP ratio may be a good indicator of the metabolic ability of TP. We found that concentration of DMU depended on the concentration of TP (Fig. 2), and the serum level of DMU correlated with that of TP in both ages of mice (Fig. 3), which suggests that the DMU/TP ratio is constant among the samples collected at same time point (data not shown). Thus, this metabolic pathway of TP to DMU still has a large capacity regardless of age. On the other hand, 1MU did not correlate with TP in both 3-week-old and 7-week-old mice, but 3MX correlated with TP in 7-week-old mice (Fig. 3). In a previous study, Konishi et al. reported that the values of the maximum reaction velocity per unit protein (Vmax) for 8-hydration of TP was much higher than that for 1-demethylation and 3-methylation of TP in mouse hepatic microsome.14) This fact means that metabolic pathway of TP to DMU has a large capacity, while metabolic pathway to 1MU and 3MX do not have enough capacity, indicating that 1MU and 3MX are not suitable for indicator of the TP-metabolizing capacity. Similar observations were also observed in the case of human.5,6) These similarities between mice and humans suggest that the DMU/TP ratio reflects the TP-metabolizing capacity in mice.

(A) TP vs. DMU in 3-week-old mice, (B) TP vs. DMU in 7-week-old mice, (C) TP vs. 3MX in 3-week-old mice, (D) TP vs. 3MX in 7-week-old mice, (E) TP vs. 1MU in 3-week-old mice, and (F) TP vs. 1MU in 7-week-old mice. Each point was plotted the concentration of TP and its metabolites at 10, 20, 30, 60, 120, and 180 min after TP administration. The linear regression of the logarithmic values was calculated using the least-squares method (A, y = 0.0287x + 0.895, r = 0.68, p < 0.001; B, y = 0.0365x + 1.07, r = 0.80, p < 0.001; C, y = 0.0005x + 0.116, r = 0.16, p = 0.367; D, y = 0.0011x + 0.036, p < 0.001; E, y = 0.0006x + 0.282, r = 0.073, p = 0.673; and F, y = 0.0037x + 0.511, r = 0.415, p = 0.012).
Age influences TP metabolism in humans.15) Thus, we compared the DMU/TP ratio in mice of different ages. TP was subcutaneously administered to 2–28-week-old mice at a dose of 45 mg/kg, and DMU/TP ratios after 60 min were higher in young mice (2-, 3-, 5-, and 7-week-old mice) than in old mice (12-, 20-, and 28-week-old mice) (Fig. 4), which suggests that TP-metabolizing capacity decreases with age. This age-dependent change in the DMU/TP ratio may be a result of changes in CYP protein expression or CYP activity because DMU is metabolized from TP by CYP 1A2, 2E1, and 3A4.16,17) Previously, Kwak et al. reported that the expression or activity of CYP 1A2 is decreased with age, and CYP 2E1 expression is maintained, and CYP 3A11, which is corresponding to CYP 3A4 in human, is slightly changed depend on age in the liver of C57/BL6 mice.18) Taken together these facts, changes in CYP protein expression may account for changes in the DMU/TP ratio in mice of different ages. However, we did not determine (i) the DMU/TP ration in under 2 weeks old and over 28 weeks old mice, and (ii) the relationship between expression/activity of CYP and the DMU/TP values. Further evidence regarding these points will be needed to be collected.

All ICR mice were subcutaneously administered TP at a dose of 45 mg/kg, and blood was collected 60 min after TP administration. The values are the mean ± S.D. (n = 4–6). * p < 0.05 vs. 7 weeks, ** p < 0.01 vs. 7 weeks.
In humans, Jackson et al. reported that TP clearance decreased with age in healthy adults.19) In addition, Vestal and colleagues found that TP clearance is higher in young people than in the elderly because of a decline in TP metabolite formation.20–22) Furthermore, we analyzed our previous data in humans5,6,23) and found that the DMU/TP ratio was highest at 0.063 ± 0.013 in patients aged 1–4 years, decreased to 0.055 ± 0.012 in patients aged 13–55 years, and decreased further to 0.041 ± 0.023 in patients aged 56–86 years. These data suggest that the effects of age on TP metabolism in humans may be evaluated using the DMU/TP ratio.
In this study, the DMU/TP ratio in mice serum decreased with age, indicating that the TP-metabolizing capacity can be estimated by measuring DMU/TP values in serum. Controlling the TP concentration in serum within a narrow range improves the effectiveness of TP therapy and minimizes the incidence of adverse effects. However, the pharmacokinetics of TP are different between individuals. In addition, endogenous and exogenous factors, such as age, disease, drug therapy, diet, pregnancy, or sex, influence TP metabolism and toxicity. Since the changes of DMU/TP values in mice can be good index for reflecting TP-metabolizing capacity, drug interaction between influencing factors and TP metabolism in human can be evaluated by studies that determine the changes of the DMU/TP value in mice. Therefore, determining TP-metabolizing capacity by measuring the DMU/TP ratio may benefit TP therapeutic drug-monitoring schedules in individuals.
This work was supported by a research grant from Keio University.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.