2019 年 42 巻 8 号 p. 1376-1383
2019 年 42 巻 8 号 p. 1376-1383
High-density lipoprotein (HDL) particles that are formed in vivo adopt a disk-shaped structure, in which the periphery of the discoidal phospholipid bilayer is surrounded by apolipoprotein. Such discoidal nanoparticles can be reconstituted with certain apolipoproteins and phospholipids and are commonly called lipid nanodisks. Apolipoprotein E (apoE), one of the HDL constituent proteins, serves as a ligand for the low-density lipoprotein (LDL) receptor. Thus, it is considered that biocompatible delivery vehicles targeting LDL receptors could be prepared by incorporating apoE as the protein component of lipid nanodisks. To enhance targeting efficiency, we designed lipid nanodisks with a large number of ligands using a peptide with the LDL receptor-binding region of apoE combined with a high lipid affinity sequence (LpA peptide). In our study, the LpA peptide spontaneously formed discoidal complexes (LpA nanodisks) of approximately 10 nm in size, equivalent to native HDL. LpA peptides on nanodisks adopted highly α-helical structures, a competent conformation capable of interacting with LDL receptors. As anticipated, the uptake of LpA nanodisks into LDL receptor-expressing cells (HepG2) was higher than that of apoE nanodisks, suggesting an enhanced targeting efficiency via the enrichment of LDL receptor-binding regions on the particle. Biodistribution studies using 111In-labeled LpA nanodisks showed little splenic accumulation and prolonged retention in blood circulation, reflecting the biocompatibility of LpA nanodisks. High accumulation of 111In-labeled LpA nanodisks was observed in the liver as well as in implanted tumors, which abundantly express LDL receptors. Thus, LpA nanodisks are potential biocompatible delivery vehicles targeting LDL receptors.
Lipoproteins are natural delivery vehicles of lipophilic compounds.1) Exchangeable apolipoproteins spontaneously assemble into discoidal complexes similar to nascent high-density lipoprotein (HDL) particles produced in our body.2,3) In such discoidal complexes called lipid nanodisks, apolipoproteins wrap around the edge of the phospholipid bilayer disk. Liposomes, which are also composed of phospholipid bilayers, have been developed as drug delivery vehicles but require surface modifications to prevent them from being recognized as foreign substances.4) Alternatively, lipid nanodisks are considered to be inherently biocompatible because they mimic the nature of HDL. Thus, lipid nanodisks present a great potential for clinical applications.5,6) Additionally, lipid nanodisks can provide a platform enabling simultaneous delivery of therapeutic and diagnostic agents for theranostic nanomedicine. However, the fundamental properties of lipid nanodisks as delivery vehicles remain to be fully elucidated.
Low-density lipoprotein (LDL) receptor, which is expressed in normal cells, especially in hepatic and steroidogenic cells as well as in tumor cells,7) is involved in a variety of physiological processes such as cholesterol homeostasis and steroid hormone synthesis. Hence, this receptor is an attractive target for pharmaceutical development.8) Apolipoprotein E (apoE) is one of the ligands for LDL receptors and can form lipid nanodisks.9) Thus, if nanodisks were produced with apoE, no special modifications would be required for targeting LDL receptors.
ApoE contains two independently folded functional domains: an N-terminal domain (22 kDa, residues 1–191) including the LDL receptor-binding region (LDLRBR) and a C-terminal domain (10 kDa, residues 216–299).10,11) Generally, a large number of ligands per particle is predicted to enhance the affinity to the target receptor.12) Assuming that the sizes of nanodisks are similar, the number of LDLRBRs per particle would increase when using a shorter form of apoE instead of the full-length apoE. Previously, essentially based on this concept, spherical nano-LDL with core lipids composed of peptides possessing an LDL receptor binding ability was developed for targeting LDL receptors.13) One of the advantages of lipid nanodisks over nano-LDL is the simple preparation method virtually obtained by mixing only. It is well known that the N-terminal domain of apoE forms lipid nanodisks.14,15) In the present study, to improve the ability of nanodisks to target LDL receptors, apoE-derived peptides containing LDLRBR were designed as nanodisk components. The obtained nanodisks were labeled with radioisotopes and fluorescent dyes simultaneously to examine their potential applications for multimodality imaging.
Dimyristoyl-phosphatidylcholine (DMPC) was purchased from NOF (Tokyo, Japan) and stored in a nitrogen atmosphere at −20°C. Rhodamine B 1,2-dihexadecanoyl-sn-glycero-3-phosphoethanolamine (Rh-PE) and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-diethylenetriaminepentaacetic acid (DTPA-PE) were obtained from Molecular Probes (Eugene, OR, U.S.A.) and Avanti Polar Lipid (Alabaster, AL, U.S.A.), respectively. Fmoc amino acid derivatives were obtained from the Peptide Institute (Minoh, Osaka, Japan) and used without further purification. Human recombinant apoE3 was obtained from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). 111InCl3 was kindly provided by Nihon Medi-Physics (Tokyo, Japan). All other reagents were special or peptide synthesis grade. Buffer used in the study was 10 mM sodium phosphate buffer (pH 7.4) unless otherwise noted.
Peptide SynthesisPrimary sequences of the synthetic peptides used in the present study are listed in Table 1. Peptide containing LDLRBR spanning residues 131–150 of apoE molecule was initially designed. Since proline-punctuated bihelical structures increase the stability of nanodisks,16) a tandem repeat peptide of LDLRBR through proline (LpL peptide) was also designed. Furthermore, a peptide containing LDLRBR attached to an 18A peptide through proline (LpA peptide) was designed. 18A peptide is one of the most well-characterized amphipathic peptides, which possess a secondary structural motif of α-helices in apolipoproteins.17) The peptides were synthesized in part by CS336X Automated Peptide Synthesizer (C S Bio, Menlo Park, CA, U.S.A.) using Fmoc chemistry and purified by HPLC. In all experiments, peptides were freshly dialyzed from 6 M guanidine hydrochloride solution into buffer before use.
| Peptide | Sequence |
|---|---|
| LDLRBR | Ac-EELRVRLASHLRKLRKRLLR-NH2 |
| LpL | Ac-EELRVRLASHLRKLRKRLLRPEELRVRLASHLRKLRKRLLR-NH2 |
| LpA | Ac-EELRVRLASHLRKLRKRLLRPDWLKAFYDKVAEKLKEAF-NH2 |
N- and C-termini were capped with an acetyl (Ac) group and an amide (NH2) group, respectively.
Solubilization of DMPC vesicles by the addition of peptides was monitored by right-angle light scattering as described.18) Stock solution of DMPC in chloroform was dried under vacuum, dispersed in buffer with vigorous vortex mixing, and subjected to five freeze-thaw cycles. DMPC vesicles (final concentrations of 50 µg/mL) were mixed with peptides (lipid/peptide 2/1 (w/w)) and turbidity clarification was followed for 10 min by measuring the scattered light intensity performed on a Hitachi F-2500 spectrophotometer (Tokyo, Japan). Excitation and emission wavelengths were set at 600 nm. The measurements were initiated immediately after the addition of peptide solution or buffer. All measurements were carried out at 24.6°C, which is the gel to liquid–crystalline phase transition temperature of DMPC vesicles.
Preparation of Lipid NanodisksLipid nanodisks were prepared by the self-assembly method, which limits the type of phospholipids that can be used but is the simplest method for preparation. Briefly, DMPC and a trace amount of Rh-PE and DTPA-PE as needed dried under vacuum was hydrated with buffer and incubated with apoE or LpA peptide at a phospholipid to polypeptide weight ratio of 2 : 1 for 2 h at room temperature. Lipid–peptide complexes were subjected to gel filtration on a Superdex 200 column (60 × 1.6 cm) and eluted with saline at a flow rate of 1 mL/min by a Biologic fast protein liquid chromatography (Bio-Rad, Hercules, CA, U.S.A.).
Physicochemical Characterization of Lipid NanodisksA dynamic light scattering (DLS) technique was used to estimate the sizes of lipid nanodisks using a Zetasizer Nano ZS (Malvern, Worcestershire, U.K.). Circular dichroism (CD) measurements were performed at a peptide concentration of 25 µg/mL on a Jasco J-820 spectropolarimeter at 37°C. The mean residual ellipticity ([θ]) was calculated using the equation [θ] = (MRW) θ/101c, where θ is the measured ellipticity in degrees, 1 is the cuvette path length (0.2 cm), c is the peptide concentration in g/mL, and the mean residue weight (MRW) is obtained from the molecular mass and the number of amino acids. Transmission electron microscopy (TEM) was used to evaluate particle morphologies essentially as described.19)
111In-Labeling of Lipid NanodisksAmong clinically useful radionuclides, 111In was selected because it is commonly used in radionuclide imaging for macromolecule.20) An aliquot of 111InCl diluted in ultrapure water (50 µL) was mixed with a volume of 50 µL of 1.0 M sodium citrate buffer (pH 5.0) and added to 900 µL lipid nanodisk suspension, resulting in a total reaction volume of 1000 µL. The labeling solution was kept at 4°C overnight to minimize any harmful influence on particle integrity and was fractionated (12 fractions of 1 mL each) by a PD-10 column to separate lipid nanodisks from free 111In. Radioactivity was measured by a PerkinElmer, Inc. 2480 WIZARD2 automatic gamma counter (PerkinElmer, Waltham, MA, U.S.A.). Taking the half-life of 111In (2.8 d) into consideration, all data were corrected for the radioactivity at the time of injection.
Cell CultureHepG2 cells, provided by the RIKEN BRC through the National Bio-Resource Project of the Ministry of Education, Culture, Sports, Science and Technology of Japan, were grown in a humidified incubator (5% CO2) at 37°C in Dulbecco’s modified Eagle’s medium (D-MEM) supplemented with 10% fetal bovine serum (FBS), 1% non-essential amino acids, 1% penicillin and streptomycin.
Cell Uptake Evaluated by RadioactivityCells were precultured in 24-well plates with medium containing normal FBS for one day. HepG2 cells were incubated with 111In-labeled lipid particles (20 µg/mL of DMPC in D-MEM) at either 4 or 37°C. After incubation, supernatant was collected and the cells were washed, dissolved in 0.2 M NaOH, and collected at 15, 30, 60, 120, and 240 min. Radioactivities of the supernatant and the cell lysate concomitant with the concentrations of cellular protein were measured. Particle uptake was expressed as the ratio of radioactivity in the cell to the initial dose (%ID) per mg of protein.
Cell Uptake Evaluated by FluorescenceCells were precultured in 6-well plates with medium containing either normal FBS or lipoprotein deficient serum (LPDS) for 2 d. HepG2 cells were incubated with Rh-PE labeled lipid particles (20 µg/mL of DMPC in D-MEM) at either 4 or 37°C for 240 min. After incubation, the cells were washed, dissolved in 0.2 M NaOH, and the concentration of cellular proteins was measured. The fluorescence intensity of Rh-PE was measured after centrifugation to remove cellular debris. Fluorescence measurements were performed at room temperature on a Hitachi F-2500 spectrophotometer (Hitachi, Tokyo, Japan). Rh-PE fluorescence spectra were recorded in a 4 × 4 mm cuvette from 560 to 650 nm at excitation wavelength of 560 nm, and the 585 nm intensity was chosen to quantify cell uptake.
AnimalsMale normal mice (ddY) and nude mice (BALB/cSlc-nu/nu) were obtained from Japan SLC (Hamamatsu, Shizuoka, Japan). The HepG2-implanted model was established by subcutaneously injecting a HepG2 cell suspension (5 × 106 cells in 100 µL of 1 : 1 PBS/Matrigel) into nude mice to make the tumor size reach approximately 10 mm in diameter. These mice were maintained in a temperature-controlled environment with free access to standard rodent chow and water. All animal experiments in the present study were approved by the Experimental Animal Research Committee at Kobe Pharmaceutical University.
Biodistribution111In-labeled lipid particles in saline (100 µL at the PC concentration of 100 µg/mL) were injected into mice via the tail veins. At 0.5–48 h post-injection, mice were sacrificed and their organs were removed. Data were calculated as the percentage of injected dose per gram of tissue (%ID/g).
Lipid and Protein AnalysisThe PC concentrations were determined using an enzymatic assay kit for choline from Wako. Concentrations of protein and peptide were determined by the Lowry procedure using bovine serum albumin (Bio-Rad) as a standard.
Statistical AnalysisFor assessing differences among the groups, ANOVA followed by Tukey’s multiple comparison test was carried out using GraphPad Prism 7 (GraphPad Software, San Diego, CA, U.S.A.). Significance level was set at p < 0.05.
To test whether apolipoprotein-derived peptides form discoidal lipid nanoparticles, we first examined the turbidity of the DMPC vesicles before and after the addition of the peptide (Fig. 1). Almost no changes in the scattered light intensity of DMPC vesicles were observed in the absence of any peptide. Addition of peptide containing only an LDLRBR sequence did not solubilize DMPC vesicles. Two α-helical segments linked together by a proline residue are known to facilitate the formation of discoidal particles.21) However, addition of the LDLRBR tandem repeat peptide (LpL peptide) did not solubilize DMPC vesicles, although the peptide showed a potential to form an α-helical structure in 2,2,2-trifluoroethanol and to bind to small unilamellar vesicles composed of 1-palmitoyl-2-oleoyl-phosphatidylcholine (POPC) (data not shown). In contrast, the LpA peptide decreased the turbidity, suggesting that this peptide can form discoidal particles.
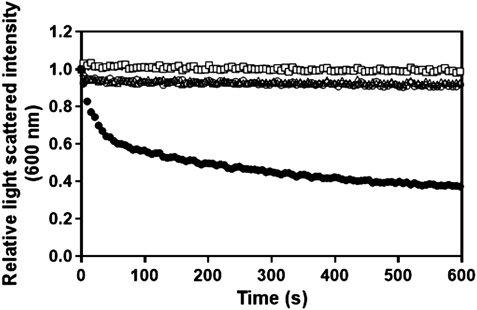
Symbols for LDLRBR peptide and no peptide are almost superimposed.
DMPC–LpA peptide complexes obtained by mixing only were subjected to gel filtration to isolate from unsolubilized vesicles and lipid-free peptides. Figure 2 shows a representative elution profile of DMPC–LpA peptide complexes. When the LpA peptide was used, the gel filtration profile was somewhat broader than that of the full-length apoE (Fig. S1). DMPC to LpA peptide weight ratio in the main peak was approximately equivalent to the initial ratio of 2 : 1. A peak near 100 min was likely attributed to lipid-free peptides.

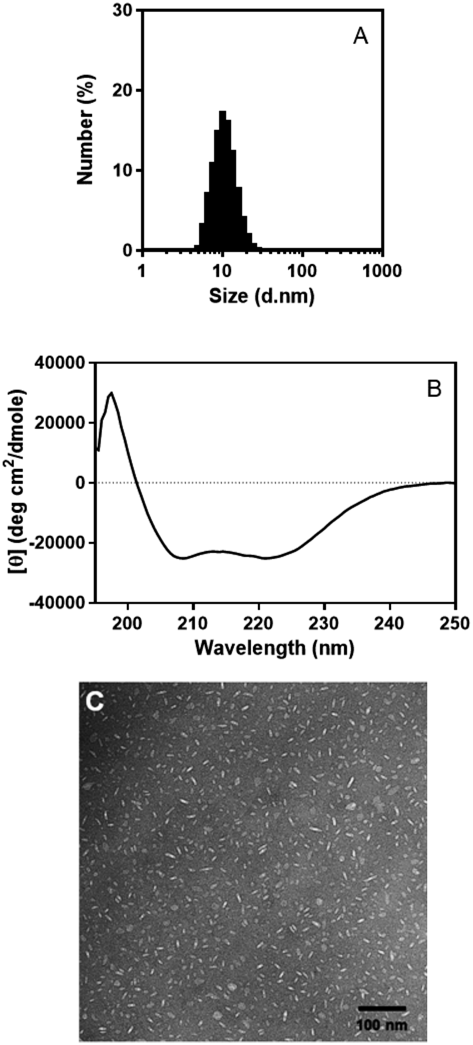
DLS analysis of the peak fraction revealed that the size of DMPC–LpA peptide complexes is approximately 10 nm, equivalent to the size of native HDL (Fig. 3A). The complexes remain stable at least 1 week when stored at 4°C. CD spectrum showed the LpA peptide adopts a highly α-helical structure at 37°C (Fig. 3B), suggesting that a large part of the peptide contributes to α-helix formation. In fact, spectral analysis using BeStSel software for secondary structure determination confirmed that the helical structure is composed by approximately 65% of the LpA peptide.22) Since LDLRBR needs to form α-helical structure in order to function as a ligand for the LDL receptor, usefulness of such complexes as delivery vehicles targeting LDL receptors is expected. Based on TEM images of DMPC–LpA peptide complexes, which demonstrated a mixture of circular and rectangular shapes corresponding with top and side views of discoidal particles, the overall morphologies are discoidal and sizes are comparable to those obtained from DLS analysis (Fig. 3C). In further experiments, fractions surrounding the peak were pooled and concentrated by ultrafiltration, a process that did not affect the particle sizes.
After incubation of LpA nanodisks with 111In, the solution was separated through a PD-10 column. In the absence of LpA nanodisks, free 111In radioactivity is detected around fraction 7 (Fig. 4A, upper). In the presence of LpA nanodisks with a trace amount of DTPA-PE, 111In radioactivity is detected in fraction 4, overlapping entirely the absorbance of the lipid marker Rh-PE (Fig. 4A, lower), and suggesting high labeling efficiency. DLS analysis showed that 111In-labeling was successfully performed without affecting the particle sizes (Fig. 4B).

We examined the time-dependent uptake of nanodisks by HepG2 cells (Fig. 5A). The uptake of LpA nanodisks at 37°C reached a plateau around 60 min, whereas the apparent uptake (cell surface binding) of LpA nanodisks at 4°C unexpectedly kept increasing over the course of 240 min, distinct from the uptake of native LDL at 4°C.23) Compared with LpA nanodisks, the uptake of apoE nanodisks was extremely low at both temperatures. These results show that cellular uptake is increased when LDLRBR is enriched on particles.

(upper: apoE nanodisks, lower: LpA nanodisks, * p < 0.05 versus 37°C FBS) (mean ± S.E.M.).
To confirm LDL receptor is involved in the enhanced cellular uptake, we examined the effect of LPDS, which is known to increase the expression of LDL receptor24) (Fig. 5B). Although apoE nanodisks uptake into HepG2 cells was extremely low compared with LpA nanodisks uptake, preincubation with LPDS medium showed a higher uptake than preincubation with FBS medium at 37°C. Similar to apoE nanodisks, uptake of LpA nanodisks was also increased by preincubation with LPDS medium. Although there might be some contributions of other receptors such as LRP to the hepatic uptake, the enhanced uptake is primarily attributed to the expression of LDL receptor. Curiously, unlike apoE nanodisks, uptake of LpA nanodisks was rather higher at 4°C than at 37°C, consistent with the time-dependent uptake results.
Biodistribution of Nanodisk Composed of LpA PeptideTable 2 summarizes the biodistribution results of 111In-labeled LpA nanodisks in normal mice. In contrast to bare liposomes, LpA nanodisks showed low accumulation in the spleen, suggesting that LpA nanodisks may avoid being recognized as foreign substances. Correspondingly, LpA nanodisks are retained in blood circulation for some time but cleared within a day. Substantial quantities of 111In were detected in the liver and the intestine, which could be due to the biliary excretion of LpA nanodisks. The biodistribution pattern was completely distinct from that of liposomes labeled in the same way (data not shown), suggesting that the labels remain in LpA nanodisks.
| Time after injection (%ID/g) | |||||
|---|---|---|---|---|---|
| 0.5 h | 3 h | 6 h | 24 h | 48 h | |
| Blood | 39.34 ± 3.96 | 12.81 ± 1.28 | 4.01 ± 0.44 | 0.26 ± 0.03 | 0.07 ± 0.01 |
| Spleen | 4.88 ± 0.74 | 4.10 ± 0.50 | 2.64 ± 0.20 | 2.12 ± 0.14 | 1.16 ± 0.08 |
| Pancreas | 1.43 ± 0.27 | 1.19 ± 0.17 | 0.73 ± 0.07 | 0.45 ± 0.03 | 0.25 ± 0.04 |
| Intestine | 1.48 ± 0.30 | 3.55 ± 0.31 | 3.64 ± 0.21 | 2.66 ± 0.23 | 0.92 ± 0.07 |
| Stomach | 1.03 ± 0.19 | 0.33 ± 0.04 | 0.31 ± 0.05 | 1.45 ± 0.56 | 0.50 ± 0.13 |
| Kidney | 7.94 ± 1.08 | 5.84 ± 0.51 | 3.96 ± 0.45 | 2.48 ± 0.15 | 2.30 ± 0.24 |
| Liver | 19.10 ± 2.87 | 41.49 ± 1.34 | 37.62 ± 3.80 | 17.64 ± 0.61 | 9.86 ± 0.69 |
| Heart | 7.12 ± 1.32 | 4.14 ± 0.31 | 1.86 ± 0.21 | 0.66 ± 0.05 | 0.43 ± 0.06 |
| Lung | 8.48 ± 1.11 | 4.74 ± 0.47 | 2.25 ± 0.21 | 0.86 ± 0.10 | 0.44 ± 0.05 |
| Muscle | 1.79 ± 0.26 | 1.43 ± 0.14 | 0.97 ± 0.12 | 0.34 ± 0.05 | 0.16 ± 0.01 |
| Bone | 3.99 ± 0.68 | 2.48 ± 0.27 | 1.70 ± 0.12 | 0.98 ± 0.12 | 0.85 ± 0.05 |
(n = 5–6).
LDL receptors are also expressed in tumor cells. LDL receptor-targeting properties of 111In-labeled LpA nanodisks were further evaluated in mice bearing HepG2 cells (Table 3). At 24 and 48 h after injection, the distribution pattern was essentially similar as in normal mice. The accumulation of 111In-labeled LpA nanodisks into tumors at 24 and 48 h were 1.18 ± 0.22 and 0.89 ± 0.14%ID/g, respectively. The tumor-to-blood ratios of 111In-labeled LpA nanodisks at 24 and 48 h were approximately 3.9 and 11, which indicates the preferential accumulation into LDL receptor-expressing tumor cells.
| Time after injection (%ID/g) | ||
|---|---|---|
| 24 h | 48 h | |
| Blood | 0.30 ± 0.05 | 0.08 ± 0.01 |
| Spleen | 2.39 ± 0.24 | 1.83 ± 0.20 |
| Pancreas | 0.17 ± 0.02 | 0.19 ± 0.03 |
| Intestine | 3.72 ± 0.33 | 1.12 ± 0.18 |
| Stomach | 0.49 ± 0.07 | 0.33 ± 0.13 |
| Kidney | 1.99 ± 0.16 | 1.71 ± 0.11 |
| Liver | 23.11 ± 2.18 | 12.75 ± 0.93 |
| Heart | 0.43 ± 0.05 | 0.36 ± 0.05 |
| Lung | 0.48 ± 0.05 | 0.33 ± 0.05 |
| Muscle | 0.13 ± 0.02 | 0.11 ± 0.01 |
| Bone | 0.52 ± 0.15 | 0.45 ± 0.12 |
| Tumor | 1.18 ± 0.22 | 0.89 ± 0.14 |
(n = 6).
The N-terminus of apoE needs to bind to lipid in order to adopt a conformation competent for interaction with LDL receptors.25) In the present study, we observed a poor ability of apoE nanodisks for LDL receptor-expressing HepG2 cell uptake. This might be because the N-terminal domain was far from the particle surface and adopted a conformation incompetent for interaction with the LDL receptor. Since the conformation of the N-terminal domain of apoE depends on the surface concentration (lipid to protein ratio) on the particle,26) efficiency of the uptake can be improved by changing the initial ratio during the preparation. In addition, since lipid composition is also a biological modulator of cellular uptake, further improvements can be expected by changing the lipid composition. Indeed, our preliminary data using POPC instead of DMPC exhibited substantially similar but entirely larger uptake of apoE nanodisks. However, as the self-assembly method using DMPC is the simplest, a balance between potential benefits and preparation labor load should be taken into account.
Nanodisks composed of only the N-terminal domain of apoE have been developed for delivery vehicles.27,28) Considering that the number of ligands on a particle determines the efficiency of LDL receptor-mediated cellular uptake, LpA nanodisks represent good candidates as delivery vehicles for targeting LDL receptor-expressing cells. The idea of designing LpA peptide to constitute nanodisks originally came from Ac-hE18A-NH2 peptide,29) in which residues 141–150 (LRKLRKRLLR) of apoE are covalently linked to 18A sequence. A cellular pathway involving heparan sulfate proteoglycan, which may inhibit LDL receptor-mediated uptake, has been proposed to mediate Ac-hE18A-NH2-bound LDL uptake,30) but no information on LpA nanodisks uptake is currently available. The present study demonstrates that the uptake is LDL receptor-mediated based on the enhanced uptake of LpA nanodisks in LPDS-containing media.
The reason for higher uptake of LpA nanodisks at 4°C than at 37°C is uncertain. It is conceivable that the secondary structure of LpA peptides on nanodisks can be altered with changes in temperature, but only minor differences in the CD spectra were observed. Enhanced drug (curcumin) uptake into tumor cells is observed with apoE nanodisks and is mainly due to drug off-loading (non-endocytic pathway) without particle internalization.31) If such a phenomenon occurs, reversible interaction of LDL receptor with LpA nanodisks would increase the non-endocytic uptake even under the conditions that no internalization takes place. At 37°C, internalization of LDL receptor decreases the receptors on the cell surface, suppressing the uptake via the non-endocytic pathway. However, it seems unlikely that DTPA-PE or Rh-PE off-load from the particles as deduced from the biodistribution results, unless the membrane surfaces of LpA nanodisks are loosely packed at 4°C. Alternatively, it is possible that the LpA nanodisks (or the labeled component) is exported from the cell after particle internalization at 37°C.
Metabolism of LpA Nanodisks in MiceUnlike liposomes recognized as foreign substances without poly (ethylene glycol) (PEG) modification, LpA nanodisks do not accumulate in the spleen. Thus, PEGylation is not required for LpA nanodisks to avoid rapid uptake from circulation by the reticuloendothelial system. Similar results were obtained with PEG-free particles mimicking lipoproteins containing core lipids.32) Since PEGylation sometimes hampers the ligand recognition on nanoparticles,33) no PEGylation is helpful for a ligand site to function efficiently. On the other hand, LpA nanodisks reasonably accumulated in the liver because LDL receptors are expressed in normal hepatocytes.
Mice have their own LDL containing apoB that can bind to LDL receptor. In plasma, compared with LDL, particles containing several molecules of apoE display higher affinity to LDL receptors.34) Thus, endogenous LDL in mice has minimal influence on the uptake of LpA nanodisks. Displacement of LpA peptides by other exchangeable apolipoproteins could occur on nanodisks.35) In addition, changes in lipid composition by phospholipid hydrolysis or lipid transfer may induce conformational changes of LpA peptides on nanodisks.36) These alterations decrease the affinity of nanodisks to LDL receptors. Further attempts to improve the stability of nanodisks are required.
Potential Applications of LpA Nanodisks as Delivery VehiclesNascent discoidal HDL particles generated in our body are converted into spherical particles by enzymatic reactions with apoA–I as an activator.37) Since apolipoproteins other than apoA–I or synthetic amphipathic peptides provide less efficient enzymatic activity,38) intravenously injected LpA nanodisks are considered to maintain the discoidal shape. It has been shown that the shape of nanoparticles affects their biological functions.39) Notably, non-spherical particles present benefits for drug delivery in terms of cellular internalization. In this context, the discoidal shape of LpA nanodisks might serve to enhance the LDL receptor-expressing cellular uptake. However, shape effects are indistinguishable because the conformation of the LpA peptide is the most important determinant for LDL receptor-mediated uptake of LpA nanodisks.
LpA nanodisks slightly accumulate in tumors, although much less than in the liver. Nanoparticles of smaller sizes present the advantage to rapidly diffuse throughout the tumor matrix.40) Especially, nanoparticles less than 40 nm can penetrate in poorly permeable tumors such as pancreatic tumors.41) In addition, reconstituted HDL composed of apoE3 has been shown to exhibit blood–brain barrier permeability.42) Although distribution of LpA nanodisks into the brain has not been observed in our study, such possibilities should be taken into consideration.
Although there are some concerns about the immune response and toxicity to be addressed, synthetic peptides are more suitable for clinical use than recombinant proteins or plasma proteins for a variety of reasons.43) In the present study, LpA peptide was used as a membrane-scaffolding molecule for nanodisks to satisfy both biocompatibility and LDL receptor-targeting efficiency. Our results suggest that LpA nanodisks can be prepared without laborious procedures and seem to be promising biocompatible delivery vehicles targeting LDL receptors. LpA nanodisks can offer a platform for delivery of multiple substances, as exemplified by two imaging modalities of radiolabeled and fluorescent phospholipids in the present report. Anticipating the ability of LpA nanodisks to load drugs,44) these particles could be utilized for theranostic applications. However, LpA peptide is not necessarily the best sequence for the reconstitution of HDL particles. Further studies are required to optimize nanoparticles for delivery vehicles including the lipid composition.
TEM observation was conducted by Dr. Shintaro Maeda and Dr. Kenji Iwasaki at the Institute for Protein Research (Osaka University), supported by the Platform Project for Supporting in Drug Discovery and Life Science Research (Platform for Drug Discovery, Informatics, and Structural Life Science) from Japan Agency for Medical Research and Development (AMED). We thank Dr. Kohei Sano, Dr. Hiroka Takase, Mr. Akira Hosotani, and Ms. Saori Ishino for their technical assistance. 111InCl3 was kindly provided by Nihon Medi-Physics.
The authors declare no conflict of interest.
The online version of this article contains supplementary materials.