2020 年 43 巻 1 号 p. 53-58
2020 年 43 巻 1 号 p. 53-58
The aim of the present study was to investigate the “chronotoxicity” of streptomycin (SM) in relation to its circadian periodicity. Male ICR mice were injected intraperitoneally with SM (780 mg/kg, one shot) one of six time points throughout the day. Mortality was monitored until 14 d after the injection and clearly differed depending on the timing of the injection (i.e., mice were more sensitive to injection during the dark phase). Moreover, when mice were administered with non-lethal doses of SM (550 mg/kg, every 24 h for 3 d, in the light phase or dark phase), the levels of nephrotoxicity indicators (blood urea nitrogen and renal levels of malondialdehyde and cyclooxygenase-2) were significantly increased by the injection in the dark phase, but not in the light phase. These results suggested that SM showed clear chronotoxicity. Our current data indicated that chronotoxicology may provide valuable information on the importance of injection timings for evaluations of toxicity and undesirable side effects.
Circadian rhythms are endogenous 24-h oscillations of the biological and behavioral processes found in all kingdoms of life. The circadian clock drives the oscillations of a diverse set of biological processes, including locomotor activity and body temperature.1) It has become clear that these circadian differences affect the frequency of diseases such as asthma and myocardial ischemia.2,3) In addition, time-dependent differences in the pharmacokinetics of medications, such as anticancer drugs, are well reported.4) Therefore, it is now important to consider chronobiological factors of medications, as it is essential to ensure the intended pharmacological action of a drug will occur. Moreover, toxicological effects should also be considered as drugs can become poison.
Our research, which we describe as “chronotoxicology,” has focused on the relationship between injection timings and the severity of cadmium (Cd) toxicity.5,6) In addition, we recently investigated the chronotoxicity of seven metals, including Ni and Cu, and found specific patterns of diurnal variation.7) These results showed the possibility that each element or compound exhibits a unique pattern of chronotoxicity. With regard to medication, several drugs, such as acetaminophen, isoniazide, and cisplatin are reported to show diurnal variations in toxicity.8–10) However, as these reports were limited to only a few drugs, there is a need to investigate the chronotoxicity of various drugs to improve quality of life.
Aminoglycoside (AGs) antibiotics are highly potent and broad-spectrum antibiotics with many desirable properties for the treatment of life-threatening infections.11) However, AGs are known to induce serious side effects, such as nephrotoxicity and ototoxicity, which are dose-limiting factors in their use. Streptomycin (SM) is one of the first aminoglycoside antibiotics used for the treatment of tuberculosis. In addition, only streptomycin is covered by insurance in Japan as a treatment for plague. In the clinical setting, SM is usually administered once daily, but the timing of administration is not a major consideration, despite reports that other AGs such as gentamycin (GM) and amikacin, show circadian variations in their pharmacokinetics and toxicology.12) To the best of our knowledge, the diurnal variation of SM-induced toxicity has not been explored, although SM is the first drug discovered for AG and an effective antibiotic against plague. We proposed that pharmacological effects may depend on the administration time of SM. In this study, we investigated the circadian variations in mouse model of SM-induced toxicity, focusing on lethal toxicity and nephrotoxicity.
Male ICR mice were purchased from Japan SLC Inc. (Shizuoka, Japan), and maintained under standard conditions of 24 ± 1°C, 55 ± 5% humidity, and a 12/12 h of light/dark cycle, with ad libitum access to water and food. Experimental treatments were performed on 7-week-old mice. At the end of the experiment (14 d after injection), surviving mice were euthanized with pentobarbital. All experiments were approved by the Institutional Animal Care and Experiment Committee of Kinjo Gakuin University (Approval No. 158).
Experimental ProtocolsFor the mortality assays, 7-week-old ICR mice were divided into six groups of five animals each. Each group was administered 780 mg/kg SM (0.1 mL/10 g body weight; FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) by intraperitoneal injection at one of six different time points (clock time; 10:00, 14:00, 18:00, 22:00, 2:00, or 6:00), described herein as zeitgeber times (ZT); ZT2, ZT6, ZT10, ZT14, ZT18, or ZT22, respectively. Injections were performed under red light during the dark period (ZT14, ZT18, and ZT22). Mortality was monitored until 14 d after injection. For the analysis of circadian variations in the renal injury, the mice received intraperitoneal injections of 550 mg/kg SM at ZT2 or ZT14 every 24 h for 3 d. The control mice received the same volume of saline. A 24 h after the final injection, the animals were euthanized and blood samples were taken for plasma collection. The collected plasma samples were stored at −80°C. The kidneys were harvested from each animal; the organ weight was measured, and the kidney were stored at −80°C or fixed in 15% neutral buffered formalin (pH 7.4).
Plasma Blood Urea Nitrogen AnalysisPlasma blood urea nitrogen (BUN) was measured by using BUN Wako Test (FUJIFILM Wako Pure Chemical Corporation) in accordance with the manufacturer’s instructions and as described previously.13)
Measurement of Total Malondialdehyde (MDA) Levels in the KidneyThe total MDA levels were measured in accordance with the manufacturer’s protocol and as described previously.6,13)
Histopathological AnalysisA portion of the left kidney was fixed in a formalin solution, dehydrated, and embedded in paraffin. Sections of 4 µm thickness were used for hematoxylin–eosin (H&E) staining and immunohistochemical analysis, as previously described.6,13) Mouse anti-cyclooxygenase-2 (COX-2) monoclonal antibody (Santa Cruz Biotechnology, Inc., CA, U.S.A.) was used as primary antibody (1 : 160 dilution). The color reaction was developed with FITC-conjugated anti-mouse IgG (MBL, Nagoya, Japan) as secondary antibody (1 : 160). Sections were counterstained with 4′,6′-diamino-2-phenylindole (DAPI; Nacalai Tesque, Inc., Kyoto, Japan).
RNA Isolation and Quantitative Real-Time PCR AssayTotal RNA was extracted from the Kidney using ISOGEN II reagent (Nippon Gene Co., Ltd., Tokyo, Japan). Five hundred nanograms of total RNA from each sample was reverse-transcribed using ReverTra Ace qPCR RT Master Mix (TOYOBO, Osaka, Japan) and then incubated for 15 min at 37°C; the reaction mixture containing synthesized cDNA was diluted three times with Tris–EDTA buffer. For the quantitative real-time PCR (qRT-PCR), 2 µL of the diluted cDNA product was amplified by using the Applied Biosystems 7300 system (Applied Biosystems, Foster City, CA, U.S.A.) in reaction mixture containing Brilliant III Ultra-Fast SYBR Green QPCR Master Mix (Agilent Technologies, Santa Clara, CA, U.S.A.) and 0.2 µM of each primer. The cycling conditions were: initial denaturation at 95°C for 3 min, followed by 40 cycles of 95°C for 5 s and 60°C for 27 s. The amount of each quantified target mRNA was normalized to the amount of β-actin. The oligonucleotide sequences of the primers used were: mouse β-actin (NM_007393) sense, 5′-GCA ACG AGC GGT TCC G-3′, and antisense, 5′-CCC AAG AAG GAA GGC TGG A-3′; mouse Cox-2 (NM_011198) sense, 5′-GCT GCA GAA TTG AAA GCC CTC-3′, and antisense, 5′-GCT CGG CTT CCA GTA TTG AG-3′.
Statistical AnalysesMultiple comparisons were made by one-way ANOVA with the Tukey–Kramer post-hoc test or two-way repeated-measures ANOVA. All statistical analyses were computed by using SPSS Statistics for Windows software (version 24.0; IBM Corporation, Armonk, NY, U.S.A.). Differences were considered statistically significant at p < 0.05.
We first investigated the effect of injection timings on SM-induced lethal toxicity. We determined the SM injection dose in accordance with the interview form of SM. Male ICR mice received a single intraperitoneal injection of 780 mg/kg SM at six time points (ZT2, ZT6, ZT10, ZT14, ZT18, and ZT22). Survival was monitored until 14 d after the injection. As shown in Figs. 1A and 1B, mice were tolerant at ZT2 and ZT6, but at showed higher susceptibility to SM-induced toxicity at ZT10, ZT14, ZT18, and ZT22. The mean survival time (MST), as estimated by Kaplan–Meier analysis was 8.4 d for ZT2 injection, 11.2 d for ZT6 injection, and 5.6 d for ZT10, ZT14, ZT18, and ZT22 injection (Fig. 1C). These results indicated that injection timings affected the severity of SM-induced toxicity. The mortality experiment was performed only once, in accordance with the principals of the 3Rs (replacement, refinement, and reduction) in animal research.
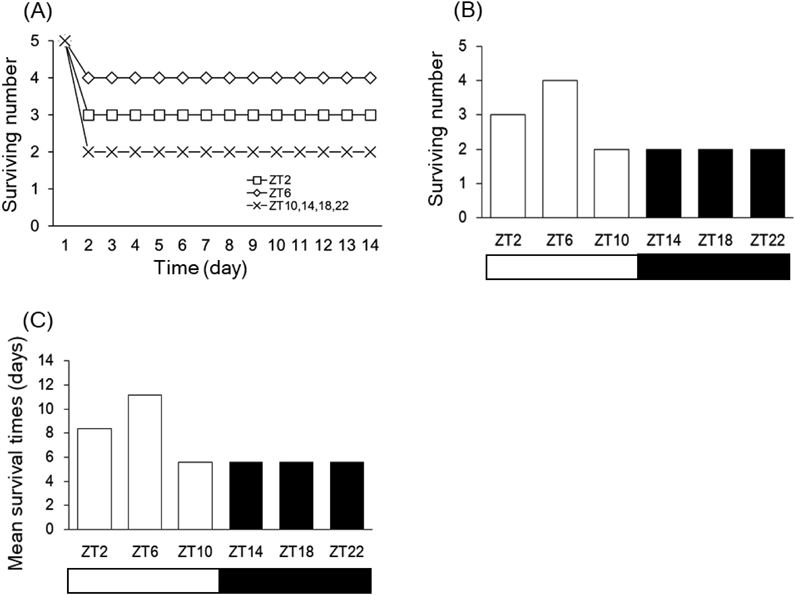
Male ICR mice (n = 5) were intraperitoneally injected with SM (780 mg/kg) at ZT2, ZT6, ZT10, ZT14, ZT18, or ZT22. Changes in the number of surviving mice over 14 d (A). Number of surviving mice on Day 14 (B). The mean survival time (MST), expressed in days, was estimated by Kaplan–Meier analysis (C).
Next, we examined the effect of injection timings on the severity of organ toxicity by using a non-lethal dose of SM (550 mg/kg). For these experiments, ZT2 and ZT14 were selected as the representative injection times. We injected SM at ZT2 or ZT14 every 24 h for 3 d. Plasma samples were collected 24 h after the final SM injections (at ZT2 or ZT14). The SM injection resulted in a slight decrease in body weight, that was comparable at both administration times (Fig. 2A). In addition, the ratio of kidney weight to body weight was not significantly changed in any group (Fig. 2B). The administration of SM led to higher plasma levels of BUN (p < 0.05), an indicator of nephrotoxicity, in the ZT14 injection group, than in the control group (Fig. 2C). In contrast, the BUN values were unaltered in the ZT2 injection group compared with the control group. Furthermore, we estimated the levels of MDA to evaluate renal oxidative stress, an indicator of SM-induced acute toxicity. We observed that SM injection increased the renal MDA levels in the ZT14 injection group, whereas no significant differences were observed in the ZT2 injection group compared with the control group (Fig. 2D).
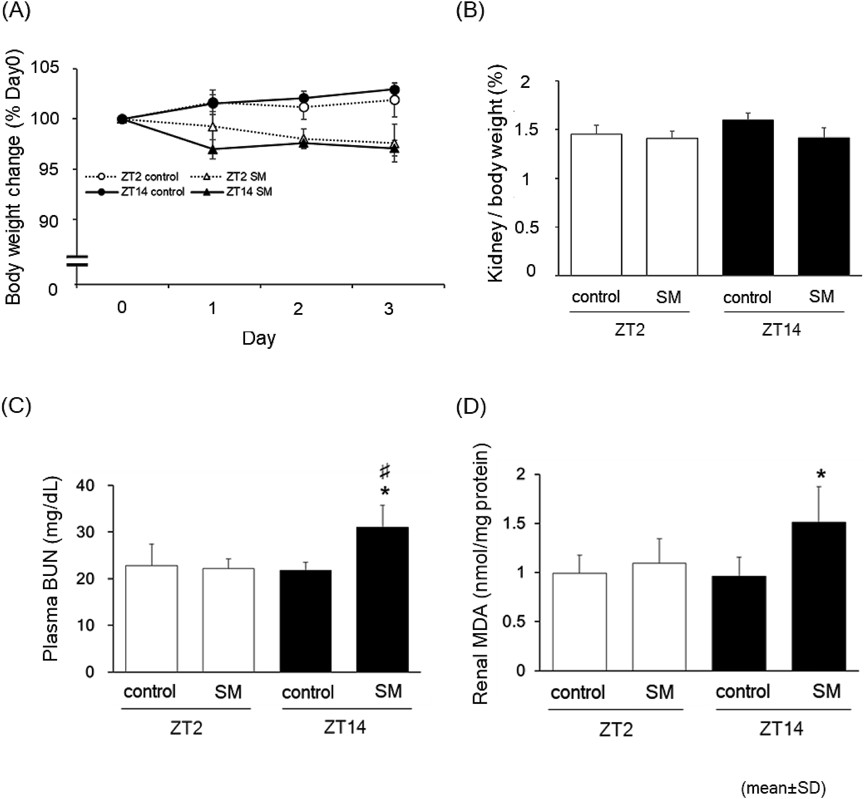
Male ICR mice (n = 6) received intraperitoneal injection of SM (550 mg/kg) at ZT2 or ZT14 every 24 h for 3 d. At 24 h after the final injection, the animals were euthanized and blood samples were taken for plasma collection. The kidneys were harvested and the organ weight was measured. The panel in (A) and (B) indicate the changes in body weight and kidney weight ratio, respectively. The levels of plasma BUN (C) and renal MDA (D) are shown. The data are represented as the mean ± standard deviation (S.D.). *, significantly different from control (p < 0.05); #, significantly different from the ZT2 SM-injected group (p < 0.05).
In addition to the measurement of biological markers (Figs. 2C, 2D), we performed a histopathological analysis of the kidneys of mice. In the control groups injection timings, normal glomerular and tubular histology were observed (Figs. 3A, 3C). Injection of SM at ZT2 resulted in slight vacuolization and necrosis in the tubules and glomerular injury (Fig. 3B). In contrast, injection at ZT14 resulted widespread tubular and glomerular injury (Fig. 3D).
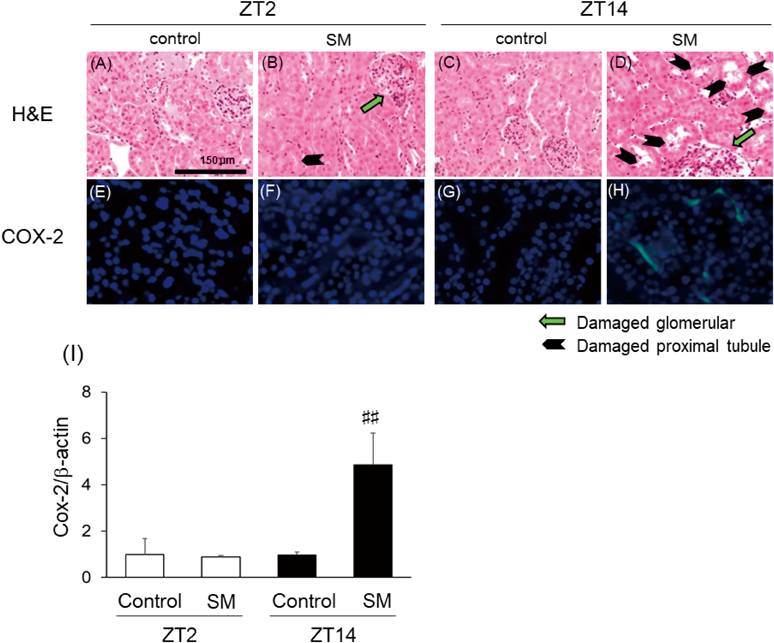
Animals were treated as described in Fig. 2 and were euthanized 24 h after intraperitoneal injection. The kidneys were harvested at necropsy and were fixed and processed according to standard staining protocols, as described in Materials and Methods. The kidney sections were stained with hematoxylin–eosin (H&E) (A–D) and COX-2 (E–H). Black arrows show injury in the renal tubules and green arrows indicate injury in the glomerulus. The expression and localization of COX-2 (green) were analyzed by using mouse anti-Cox-2 monoclonal antibody and anti-mouse IgG-FITC. The nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). The renal mRNA expression of Cox-2 (I) is shown. The mRNA levels of β-actin was used as the internal control. The data are presented as the mean ± S.D. ##, significantly different from the ZT2 SM-injected (p < 0.01).
Finally, to assess SM-induced damage, we investigated the changes in the protein expression of COX-2, as SM is known to induce the inflammatory response in the kidney. We performed immunostaining for COX-2 in the kidney sections, and observed no expression of COX-2 in the ZT2 SM-injected group, but clear COX-2 staining in the nucleus and cytoplasm of kidney sections from the ZT14 SM injection group (Fig. 3F). To confirm the immunostaining results, we performed a real-time PCR assay (Fig. 3I). These results were consistent with the immunostaining results.
In this study, we have investigated the circadian variations in a mouse model of SM-induced toxicity. We showed that lethal toxicity and nephrotoxicity varied with injection timings, and that the mice were sensitive to SM-induced toxicity during the dark-phase injection. We have previously shown that mice are sensitive to Cd toxicity (lethal toxicity and hepatotoxicity) when injected during the light-phase.5,6) The evidence suggests that injection timing may influence medication-induced undesirable side effects.
In our previous chronotoxicological study, we showed that mice exhibited increased susceptibility to Cu- and Zn-induced toxicity during the dark-phase, similar to the susceptibility to SM-induced toxicity, whereas mice were sensitive to Cd and Ni during the light-phase.6,7) We speculate that the target organ provides important information about the chronotoxicity. For example, the liver and testis are the target organs of Cd-induced acute toxicity,6,14) and the liver and kidney show Cu-induced acute toxicity.15) Therefore, it is possible that chronotoxicity is mediated mainly by the target organs.
AGs are frequently prescribed as first and second line drugs in a variety of clinical situations. However, many reports indicate that AGs-induce nephrotoxicity.16–18) Most of the available data on the mechanisms of AG-induced nephrotoxicity has been obtained on GM in animal models and cell culture studies. AG-induced nephrotoxicity is mainly caused by tubular cytotoxicity. The administration of GM in experimental animals induced tubular damage by necrosis and apoptosis in renal epithelial cells.19,20) Similar effects have also been observed in cell culture studies.21,22) The present study H&E staining results showed that mild tubular damage was observed with ZT2 injection, but more severe renal damage, i.e., widespread injury in the tubules and glomerulus, was observed with ZT14 injection. Although our investigation did not evaluate necrosis, apoptosis, or specific markers for proximal tubules injury such as β2-microglobulin and kidney injury molecule-1 (KIM-1), changes in injection timings resulted in altered nephrotoxicity. Oxidative stress is a key factor in AGs-induced nephrotoxicity as co-administration with anti-oxidants protects against AGs-induced renal damage.23,24) Moreover, an association with inflammatory responses (cell infiltration and increased cytokine production) has also been reported for AGs-induced nephrotoxicity in experimental animals.23,24) As the inflammatory response is initially induced as a defense and repair mechanism, the inhibition of this response can protect against AGs-induced nephrotoxicity. In our current study, the injection of SM at ZT14 increased levels of renal MDA and COX-2 expression compared with a saline-injected control, whereas these parameters were comparable with the saline-injected control when SM was injected at ZT2. These results suggested that oxidative stress induced in the kidney may be more intense following SM injection at ZT14.
This raises the question of which biological factors (or biological conditions) are responsible for this time-dependent drug sensitivity. We have previously shown that levels of antioxidants, such as metallothionein, were higher in the light phase (ZT2) than in the dark phase (ZT14), suggesting that these diurnal differences may regulate nephrotoxicity. In addition, the cytotoxicity induced by GM occurs in the organs in which the drug accumulates. In the kidney, for example, GM accumulates in the epithelial cells of the cortex, mainly in the proximal tubules, distal tubules and collecting ducts.25,26) This result suggests that endocytosis-related receptors such as megalin may be involved in GM accumulation.27) Further, determination of the blood concentration of the drug is necessary for therapeutic drug monitoring. The blood concentration of drug is also influenced by food intake. Therefore, further investigations, such as examination of the megalin level and SM concentration in the kidney and blood in addition to excluding food intake, are needed to elucidate effect of injection timing on SM-induced nephrotoxicity.
The chronotoxicity of antibiotics such as GM and tobramycin was first reported more than 20 years ago.28,29) These reports showed that the induced toxicity was more serve when administrated during the rest times compared with activity timing. This result was not consistent with our current data, despite the use of the same type of antibiotics. However, we have data for carbon tetrachloride and acetaminophen, that show opposite chronotoxicity patterns despite both compounds inducing hepatotoxicity.30,31) These results suggest that it is important to determine the chronotoxicity pattern of individual chemicals.
In conclusion, we demonstrated the chronotoxicity of SM administration. Mice were sensitive to SM-induced toxicity when exposed during the dark phase (ZT14 injection). We propose that chronotoxicology of drugs should be considered when determining any undesirable side effects.
This work was supported by Kinjo Gakuin University Research Grant and a Grant-in-Aid for fundamental research (N-F29-02) from National Institute of Occupational Safety and Health, Japan (JNIOSH).
The authors declare no conflict of interest.