2020 年 43 巻 1 号 p. 179-183
2020 年 43 巻 1 号 p. 179-183
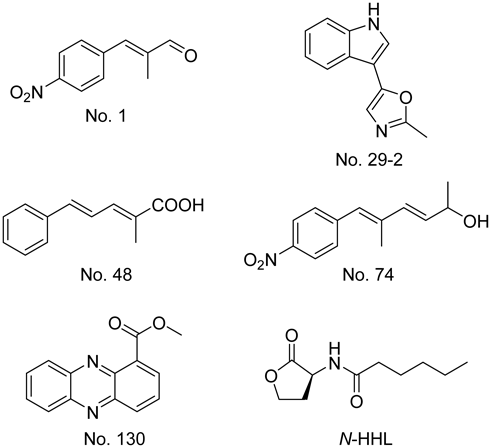
Quorum sensing (QS) is a microbial signaling system that regulates the expression of many virulence genes. Herein, we studied five compounds—No. 1: (E)-2-methyl-3- (4-nitro-phenyl)-acrylaldehyde; No. 29-2: pimprinine [5-(1H-indol-3-yl)-2-methyloxazole]; No. 48: (2E,4E)-2-methyl-5-phenyl-2,4-pentadienoic acid; No. 74: (3E,5E)-5-methyl-6-(4-nitrophenyl)-hexa-3,5-dien-2-ol; and No. 130: methyphenazine-1-carboxylate—derived from an actinomycete metabolite library. These compounds were confirmed to be QS inhibitors that reduced violacein production in Chromobacterium violaceum CV026. Additionally, compounds No. 1, No. 74, and No. 130 significantly reduced fluorescent pigment production in Pseudomonas aeruginosa ATCC 27853.
Quorum sensing (QS) is a signal transduction system in bacteria that is mediated by substances excreted from bacterial cells into the environment.1) This system regulates the expression of many virulence genes, including those encoding proteins for biofilm formation,2) swarming motility,3) antibiotic biosynthesis,4,5) and virulence factor production.6,7) A novel strategy to control microbial infections involves the use of agents that inhibit microbial virulence without inhibiting growth, because less selection pressure would generate less microbial resistance.8,9)
The disruption of the QS system in pathogenic Burkholderia cepacia and Burkholderia pseudomallei results in reduced pathogenicity in rat and mouse models of infection.10,11) At concentrations below the minimum inhibitory concentration (MIC), erythromycin inhibits biofilm formation in Pseudomonas aeruginosa.12) LED209 is a non-toxic compound that does not inhibit pathogen growth but markedly inhibits the virulence of several pathogens in vitro and in vivo.13) Therefore, compounds that inhibit QS have great potential for use in the treatment of bacterial infectious diseases.
Based on these novel “anti-infective drugs,” we previously established a screening system using Chromobacterium violaceum CV02614) to identify QS inhibitors produced by actinomycetes. Piericidin E, a novel compound, was recently discovered in Streptomyces sp. TOHO-Y209 culture broth.15) Furthermore, Streptomyces sp. TOHO-M025 was found to produce novel compounds maniwamycins C–F that inhibited QS in C. violaceum CV026.16)
In this study, the QS inhibition activity of 322 samples from our actinomycete metabolite library was evaluated using a screening system with C. violaceum CV026. In addition, these active compounds were tested for their effect on fluorescent pigment production by P. aeruginosa.
C. violaceum CV02614) was cultured in Luria Broth [LB; 1.0% Bacto tryptone (Becton, Dickinson and Company, Sparks, MD, U.S.A.), 0.5% Bacto yeast extract (Becton, Dickinson and Company), and 0.5% NaCl (Wako Pure Chemical Corporation, Osaka, Japan), pH 7.2] at 27°C. P. aeruginosa ATC C 27853,17) a quality control strain for dilution antimicrobial susceptibility tests, was cultured overnight in Nutrient Broth (NB; Eiken Chemical Co., Ltd., Tochigi, Japan) at 37°C.
Screening SamplesIn total, 322 samples from our actinomycete metabolite library, consisting of compounds obtained from actinomycete cultures and synthesized as partial structures of actinomycete metabolites, were used for screening. These compounds were stored by Professors Yasumasa Koyama and Fumio Kato, who studied metabolites (including antibiotics) produced by actinomycetes 40 to 50 years ago. The structures of the active samples were confirmed by 1H-NMR (500 MHz) spectroscopy (JNM-ECA500, JEOL Ltd., Tokyo, Japan) and mass spectrometry (LCMS-8040, Shimadzu Co., Kyoto, Japan).
QS Inhibition AssayThe microtiter plate assay was performed for QS inhibition screening, according to our previous study.16) In the checkerboard assay, 0.0625, 0.125, 0.25, or 0.5 mg/mL of the test compound and 0.008, 0.04, or 0.2 mg/mL of N-hexanoyl-D-homoserine lactone (N-HHL; Santa Cruz Biotech. Co., Santa Cruz, CA, U.S.A.) were added to each well.
Effect of Compounds on Fluorescent Pigment Production by P. aeruginosaIn each well of a 96-well cell culture plate (CELLSTAR® 96 well plates No. 655180, Greiner Bio-One GmbH, Frickerhansen, Germany), 200 µL of NB containing 0.5 µL of P. aeruginosa ATC C 27853 culture broth and 0.5 mg/mL of the test compound were added. The microtiter plate was incubated at 37°C for 24 h with shaking. Equal volumes of the culture broth (100 µL) and dimethyl sulfoxide (DMSO) (100 µL) were added to each well of a 96-well black plate (Nunc™ F96 MicroWell™ Black Polystyrene Plate No. 137101, Thermo Fisher Scientific, Roskilde, Denmark), and fluorescence intensity at an absorption wavelength of 470 nm and excitation wavelength of 365 nm was measured using a multimode plate reader EnSpire2300 (PerkinElmer, Inc., MA, U.S.A.).
Antibacterial Activity and Bacterial Growth RateThe antibacterial activities of the compounds for C. violaceum CV026 and P. aeruginosa ATC C 27853 were evaluated by determining MICs using the microbroth dilution method with Mueller–Hinton medium (Becton, Dickinson and Company). The test compounds were serially diluted and added to the growth medium in 96-well plates. Each well was inoculated with the tested bacterial strains and incubated at 27 or 37°C for 20–24 h. Bacterial growth was evaluated by OD600 measurement. The growth rates of both strains were also evaluated under pigment production culture conditions through OD600 measurement in 96-well plates. C. violaceum CV026 was cultured in LB soft agar ± test compound (0.5 mg/mL) in the absence of N-HHL.
The QS inhibitory activity was determined using C. violaceum CV026 and 322 samples from the actinomycete metabolite library. At a concentration of 1 mg/mL, five compounds reduced violacein production in C. violaceum to approximately half or less of its original value. As the structural formulae of other compounds were indeterminable in some samples from the library, the structures of these five compounds were confirmed (data not shown). These five compounds were identified as: No. 1, (E)-2-methyl-3-(4-nitro-phenyl)-acrylaldehyde; No. 29-2, pimprinine [5-(1H-indol-3-yl)-2-methyloxazole]; No. 48, (2E,4E)-2-methyl-5-phenyl-2,4-pentadienoic acid; No. 74, (3E,5E)-5-methyl-6-(4-nitrophenyl)-hexa-3,5-dien-2-ol; and No. 130, methyphenazine-1-carboxylate (Fig. 1).
A checkerboard assay was performed to determine if these compounds competed with N-HHL, which was used as an inducer of QS (Fig. 2). Violacein production was completely suppressed by No. 1 and No. 74 at 0.25 mg/mL and by No. 130 at 0.5 mg/mL. A concentration-dependent competitive inhibition of N-HHL was observed below these concentrations for No. 1, No. 74, and No. 130. In contrast, No. 29-2 and No. 48 showed a concentration-dependent partial inhibition of N-HHL; moreover, violacein production was not completely suppressed, even at 0.5 mg/mL.

Violacein production is plotted as the concentration of N-HHL for each QS inhibitor concentration (circle: 0 mg/mL, rhombus: 0.0625 mg/mL, triangle: 0.125 mg/mL, asterisk: 0.25 mg/mL, and square: 0.5 mg/mL).
The effects of these five compounds on fluorescent pigment production by P. aeruginosa were examined (Fig. 3). At 0.5 mg/mL, No. 1, No. 74, and No. 130 decreased fluorescent pigment production by 69, 70, and 67%, respectively. In contrast, No. 29-2 and No. 48 increased fluorescent pigment production by 14 and 11%, respectively, relative to the control.

Each compound (0.5 mg/mL) was added to 200 µL NB with 0.5 µL pre-culture broth. After incubation at 37°C for 24 h, the excitation spectra for the fluorescent pigment in culture broth was measured at an emission wavelength of 470 nm and excitation wavelength of 365 nm. Error bars represent the standard error of the mean for three biological replicates. * indicates a significant increase compared to the control (no compound) at p < 0.05. † indicates a significant decrease compared to the control at p < 0.05.
The antibacterial activities of these compounds against C. violaceum and P. aeruginosa were tested based on the Clinical and Laboratory Standards Institute (CLSI) methods using Mueller–Hinton medium. No. 1 showed no antibacterial activity against any strain at concentrations from 1 to 512 µg/mL. However, P. aeruginosa culture turbidity decreased by 42% at 1024 µg/mL No. 1. The other compounds showed no antibacterial activity, even at 1024 µg/mL; moreover, none showed growth–inhibitory effects against C. violaceum.
The 24-h growth curve for each of these bacteria was determined under pigment production conditions to confirm the effects of these compounds (Fig. 4). The C. violaceum CV026 growth curves with No. 1 and No. 29-2 were similar to that of the control from 0 to 24 h, whereas No. 74 slightly suppressed C. violaceum CV026 growth after 12 h. C. violaceum CV026 treated with No. 48 and No. 130 grew better than the control during the intermediate-to-late growth stage (6–12 h). After 24 h, the OD600 of the C. violaceum CV026 culture treated with No. 48 reached 0.95, whereas that treated with No. 130 reached 0.73, similar to that of the control. In contrast, the growth rate of P. aeruginosa ATC C 27853 was not affected at all by these compounds. During the initial growth stage (0–8 h), the OD600 of the cultures treated with the test compounds as well as the control was increased. However, after 8 h, the cells began to aggregate and the OD600 varied. This strain grew planktonically up to 8 h and aggregated thereafter. The OD600 of the cultures treated with the test compounds was similar to that of the control after 24 h.
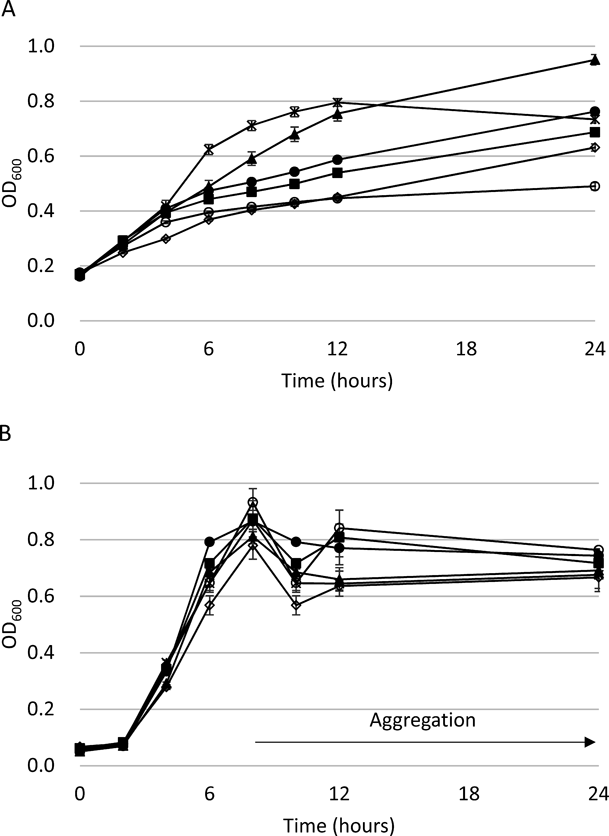
C. violaceum CV026 was cultured in LB soft agar ± compound without N-HHL at 27°C. P. aeruginosa ATCC 27853 was cultured in NB ± compound at 37°C with shaking. After 8 h, P. aeruginosa ATCC 27853 cells began to aggregate in each well. Error bars represent the standard error of the mean for three biological replicates.
None of the five compounds that reduced violacein production in C. violaceum have been previously reported as QS inhibitors. No. 1, (E)-2-methyl-3-(4-nitro-phenyl)-acrylaldehyde, was previously reported as a metabolite produced by Streptomyces luteoreticuli. Moreover, unsaturated ketone derivatives of sphenyl-acrolein have been shown to exhibit antimicrobial activity.18) An unsaturated ketone derivative of No. 1, synthesized by Gasha et al., inhibited the growth of Trichophyton mentagrophytes.19) No. 29-2, pimprinine [5-(1H-indol-3-yl)-2-methyloxazole]—a metabolite produced by Streptomyces pimprina—20) was reported to exhibit anti-tuberculosis activity.21) No. 48, (2E,4E)-2-methyl-5-phenyl-2,4-pentadienoic acid, was synthesized by Koyama et al. and used for structure determination of luteoreticulin produced by S. luteoreticuli.18) No. 74, (3E,5E)-5-methyl-6-(4-nitrophenyl)-hexa-3,5-dien-2-ol, was synthesized as a derivative of aureothin, which was isolated as a by-product of the antibiotic aureothricin from Streptomyces thioluteus.22) No. 130, methyphenazine-1-carboxylate, was isolated as a metabolite from S. luteoreticuli in 1971.23) Recently, No. 130 was reported as a metabolite produced by Pseudomonas chlororaphis and strains of the genus Kitasatospora; moreover, the antifungal and anti-Gram-positive-bacterial activities of No. 130 were demonstrated.24,25)
As No. 1, No. 74, and No. 130 act competitively with N-HHL, an inducer of QS, in C. violaceum, these compounds can inhibit the activity of the transcription factor CviR, which is induced by N-HHL in C. violaceum CV026. Interestingly, the structures of No. 1 and No. 74 are very similar, and the sub-structures of both compounds consist of 1-nitro-4-(1-(1E)-propenyl)-benzene. Moreover, in our study, these three compounds significantly reduced fluorescent pigment production in P. aeruginosa. On the contrary, all five compounds showed no effect on the production of the green pigment pyocyanin, which is positively regulated by the RhlR–RhlI QS system.26)P. aeruginosa ATC C 27853 did not produce pyocyanin for up to 24 h; however, pyocyanin was detected after 2 d. There was no difference in pyocyanin production on the next day, when fluorescent pigment production was observed (data not shown). The QS response of P. aeruginosa primarily depends on two transcriptional regulators of the LuxR family, LasR and RhlR.27) Moreover, the active site of LasR is structurally similar to that of CviR.28) Therefore, it is presumed that No. 1, No. 74, and No. 130 bind to the LasR protein of P. aeruginosa. The production of a fluorescent pigment pyoverdine by P. aeruginosa is reduced in the lasI/lasR mutant29) and enhanced by the cell-signaling protein PqsA.30) The transcription of pqsA is induced by the third transcriptional regulator PqsR, which is positively regulated by the LasR/N-3-oxododecanoyl-D-homoserine complex.31) However, as the growth curves of C. violaceum CV026 treated with No. 74 and No. 130 were slightly different from that of the control, the QS inhibition system of these active compounds including No. 1 should be further investigated. Conversely, No. 29-2 and No. 48 slightly increased fluorescent pigment production in P. aeruginosa. As No. 29-2 and No. 48 only partially competed with N-HHL in C. violaceum, CviR function might have been partially inhibited. The fluorescence intensity (excitation 365 nm, absorption 470 nm) of the compounds No. 29-2 and No. 48 was not detected (data not shown). However, as the increase in fluorescent pigment production was very less, this phenomenon may have been influenced by growth variations caused by cell aggregation during culture.
Several new compounds, such as piericidin E and maniwamycins C–F, have been discovered in actinomycete culture broths and were used in our QS inhibitor screening study.15,16) In this study, five compounds from a library of actinomycete metabolites and their derivatives were identified as QS inhibitors against C. violaceum. Additionally, they displayed positive or negative effects on fluorescent pigment production by P. aeruginosa. Thus, these QS inhibitors can be potential lead compounds for the creation of more potent and specific QS inhibitors for further studies of QS systems.
We thank Dr. Tsukasa Ikeda at Utsunomiya University for kindly providing C. violaceum CV026. This work was supported by Private University Research Branding Project from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan.
The authors declare no conflict of interest.