2020 年 43 巻 11 号 p. 1792-1798
2020 年 43 巻 11 号 p. 1792-1798
Xanthine and hypoxanthine are intermediate metabolites of uric acid and a source of reactive oxidative species (ROS) by xanthine oxidoreductase (XOR), suggesting that facilitating their elimination is beneficial. Since they are reabsorbed in renal proximal tubules, we investigated their reabsorption mechanism by focusing on the renal uric acid transporters URAT1 and GLUT9, and examined the effect of clinically used URAT1 inhibitor on their renal clearance when their plasma concentration is increased by XOR inhibitor. Uptake study for [3H]xanthine and [3H]hypoxanthine was performed using URAT1- and GLUT9-expressing Xenopus oocytes. Transcellular transport study for [3H]xanthine was carried out using Madin–Darby canine kidney (MDCK)II cells co-expressing URAT1 and GLUT9. In in vivo pharmacokinetic study, renal clearance of xanthine was estimated based on plasma concentration and urinary recovery. Uptake by URAT1- and GLUT9-expressing oocytes demonstrated that xanthine is a substrate of URAT1 and GLUT9, while hypoxanthine is not. Transcellular transport of xanthine in MDCKII cells co-expressing URAT1 and GLUT9 was significantly higher than those in mock cells and cells expressing URAT1 or GLUT9 alone. Furthermore, dotinurad, a URAT1 inhibitor, increased renal clearance of xanthine in rats treated with topiroxostat to inhibit XOR. It was suggested that xanthine is reabsorbed in the same manner as uric acid through URAT1 and GLUT9, while hypoxanthine is not. Accordingly, it is expected that treatment with XOR and URAT1 inhibitors will effectively decrease purine pools in the body and prevent cell injury due to ROS generated during XOR-mediated reactions.
Xanthine and hypoxanthine are important intermediates in purine metabolism, and they are subsequently converted to uric acid by xanthine oxidoreductase (XOR). Although their physiological roles are not fully understood, XOR knockout mice showed renal failure after birth, and most died within the first month.1) In the same literature, blood concentrations of xanthine and hypoxanthine in 2 weeks old mice were about 0.4 and 0.2 mg/dL, respectively, but rapidly decreased to below the detection limit (<5 µg/dL). Therefore, xanthine and hypoxanthine might play important roles in the early stage of kidney development.1) Furthermore, during metabolic reactions by XOR, they could cause cytotoxicity by generating reactive oxygen species (ROS), resulting in cell injury.2) On the other hand, it has been reported that hypoxanthine is reused in the salvage pathway for ATP synthesis, while xanthine is not utilized.3) Although their blood concentrations are usually very low in healthy subjects, their plasma concentrations increase when XOR inhibitors are used in treating hyperuricemia and gout subjects.4) They are metabolized to uric acid when the plasma concentration of XOR inhibitor is below the effective concentration, forming uric acid pools. Our recent study also suggested that the supply of uric acid from hepatocytes to blood is more significantly affected by hepatic metabolism and the transport of xanthine and hypoxanthine than uric acid itself, suggesting that the disposition of hypoxanthine and xanthine influences serum uric acid level.5) A previous clinical study showed that xanthine and hypoxanthine are reabsorbed in proximal tubular cells after glomerular filtration with fractional excretion (FE) of 20–30%, which was obtained by dividing their urinary excretion clearance by creatinine clearance.6) Therefore, reducing their reabsorption is considered an effective strategy for the treatment of hyperuricemia, and it prevents cell damage due to ROS generated during XOR-mediated reactions. However, their renal reabsorption mechanism remains unclear.
A previous study showed that hypoxanthine was a substrate of equilibrative nucleoside transporter (ENT)1, ENT2, and equilibrative nucleobase transporter 1 (ENBT1), but not a substrate of concentrative nucleoside transporter (CNT)1, CNT2, and CNT3.7–9) There is no report on the membrane transport mechanism of xanthine. Since ENT1 and ENT2 are predominantly localized on the basolateral membrane in mice renal proximal tubule cells,10) and ENBT1 is rarely expressed in the kidney unlike in the liver,9) uptake of xanthine and hypoxanthine at the apical membrane of the renal epithelial cells is hardly accounted for by the nucleoside and nucleobase transporters mentioned above.
Xanthine and hypoxanthine are uric acid analogues with pKa values of 7.53 and 8.72, respectively. They, as well as uric acid that has a pKa value of 5.8, are weakly acidic compounds. Previously, we reported that uric acid analogues (1-methyluric acid, 1,3-dimethyluric acid, oxypurinol, and 6-thiouric acid) were substrates of uric acid reabsorptive transporter 1 (URAT1).11) Therefore, it was hypothesized that uric acid transporter are involved in the renal reabsorption mechanism of xanthine and hypoxanthine. The urinary excretion of uric acid is determined by glomerular filtration, tubular reabsorption, and secretion, which are mediated by specific transporters URAT1,12,13) glucose transporter 9 (GLUT9),14,15) organic anion transporters (OAT1–4),16–19) Na+-phosphate cotransporter 1 and 4 (NPT1 and 4),20,21) breast cancer resistance protein (BCRP),22,23) and multidrug resistance associated protein 4 (MRP4).19) Among them, URAT1 and GLUT9, which are localized at the apical and basolateral membranes of renal proximal tubular cells, respectively, are responsible for renal reabsorption of uric acid.12,24) If xanthine and/or hypoxanthine are reabsorbed by URAT1 and GLUT9, URAT1 inhibitors such as benzbromarone and dotinurad,25–27) could reduce their blood concentration when XOR inhibitor is administered.
In the present study, we investigated whether xanthine and hypoxanthine are substrates of URAT1 and GLUT9. Furthermore, the effect of URAT1 inhibitor on their renal clearance in rats and their transport in artificial renal cells expressing URAT1 and GLUT928) were examined.
[14C]Uric acid (1.96 TBq/mol) and [3H]xanthine (370 TBq/mol) were purchased from Moravek Biochemicals (Brea, CA, U.S.A.). [3H]Hypoxanthine (740 TBq/mol) was purchased from Muromachi Chemicals (Tokyo, Japan). Dotinurad and topiroxostat were obtained from Fuji Yakuhin Co., Ltd. (Saitama, Japan). All other chemicals were commercial products of reagent grade.
Uptake Study by Xenopus OocyteXenopus laevis frogs were obtained from Kato-S-Science (Chiba, Japan). Uptake of xanthine and hypoxanthine by URAT1 and GLUT9 isoform 1 was conducted with Xenopus laevis oocytes by culturing them for three days after microinjecting synthesized complementary RNA (cRNA) of URAT1 or GLUT9 isoform 1 as previously described.11,29) Briefly, defoliculated oocytes were injected with 50 nL of water containing 12.5 ng of cRNA of human URAT1 or GLUT9 and were cultured for 3 d in modified Barth’s solution (MBS, 88 mM NaCl, 1 mM KCl, 2.4 mM NaHCO3, 0.82 mM MgSO4, 0.33 mM Ca(NO)3, 0.41 mM CaCl2 and 10 mM N-(2-hydroxyethyl)piperazine-N′-2-ethanesulfonic acid (HEPES) adjusted to pH 7.4 with NaOH). To initiate uptake reaction, the oocytes were pre-incubated in ND96 buffer (96 mM NaCl, 2 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, and 5 mM HEPES, pH 7.4) at 25 °C for 15 min and were incubated with Cl− free (replaced with gluconate of ND96 buffer) uptake buffer containing test radio-labeled substrate at 25 °C for the designated time. Uptake was terminated by washing the cells three times with ice-cold uptake buffer. As a control, uptake by oocytes that were injected with the same volume of water was measured with the same procedures.
Transcellular Transport Assay Using Madin–Darby Canine Kidney (MDCK)II Cells Co-expressing URAT1 and GLUT9MDCKII cells expressing URAT1 alone, GLUT9 isoform 1 alone, and co-expressing URAT1 and GLUT9 isoform 1 cells were constructed as described previously.28) Cells were cultured on Transwell® filter membrane inserts (3.0 µm pore size, 1.12 cm2 surface area, BD, Franklin Lakes, NJ, U.S.A.) at a density of 2.0 × 105 cells/cm2 for 5 d before transport study. The transport was initiated by adding 500 µL of chloride-free transport buffer (136.7 mM Na-gluconate, 0.952 mM Ca-gluconate, 5.36 mM K-gluconate, 0.441 mM KH2PO4, 0.812 mM MgSO4, 0.383 mM Na2HPO4, 25 mM D-glucose and 25 mM HEPES, pH 7.4) containing test substrates to the apical side at 37 °C. Aliquots of the buffer were withdrawn from the basolateral side at the indicated time points. The apparent permeability (Papp, cm/s) of test substrates was calculated using the following equation: Papp = (dQ/dt)/(A × C0), where Q, C0 and A are the amount of transported substrate, the initial concentration of test substrates on the donor side, and the surface area of the membrane, respectively.
Pharmacodynamic StudyAll animal studies were approved by the Kanazawa University Institutional Animal Care and Use Committee (Permit No. AP-183955) and performed in accordance with the university guidelines. Male Wistar rats (8–10 weeks) were purchased from Japan SLC (Shizuoka, Japan). They were housed three per cage with free access to commercial chow and tap water, and were maintained on a 12/12 h dark/light cycle in an air-controlled room (temperature, 24.0 ± 1 °C; humidity, 55 ± 5%). Pharmacodynamic studies were carried out in rats as previously described with slight modifications.11) The rats were anesthetized with pentobarbital (50 mg/kg), and their bladders were cannulated using polyethylene tubes (inside 0.5 mm, outside 0.8 mm). XOR inhibitor topiroxostat (1 mg/kg) alone or topiroxostat and URAT1 inhibitor dotinurad (100 mg/kg) were orally administrated to rats. Then, blood was drawn from the jugular vein at 0, 15, and 30 min, and centrifuged at 3000 × g for 10 min at 4 °C to obtain plasma. Urine was collected at 0–30 min. All samples were stored at −80 °C until the measurement.
Measurement of CompoundsHypoxanthine, xanthine, and uric acid were measured using radiolabeled compounds or by HPLC. The radioactivity was measured with a liquid scintillation counter after addition of liquid scintillation cocktail (Nacalai Tesque, Kyoto, Japan). Moreover, the amount of xanthine and uric acid by animal study was determined with an HPLC system (Alliance 2690/UV/VIS Detector 486, Waters, Milford, MA, U.S.A.). The HPLC analysis was performed using Mightysil RP-18 GP 5 mm (250 × 4.6 mm, Kanto Chemical Co., Tokyo, Japan) as an analytical column maintained at 30 °C. The mobile phase was composed of 0.5% acetic acid, delivered at a flow rate of 1 mL/min. Xanthine and uric acid was detected at 260 and 284 nm, respectively. The detection limit of xanthine and uric acid was 0.01 and 0.05 mg/dL, respectively.
Concentration of creatinine in rat plasma and urine was measured by means of LC-MS/MS method as described previously.11) Briefly, the amount of creatinine was determined with a LCMS-8050 triple quadrupole LC-MS/MS (Shimadzu, Kyoto, Japan) coupled to an LC-30A system (Shimadzu) at 40 °C. Atlantis® hydrophilic interaction liquid chromatography (HILIC) Silica column (5 µm, 2.1 × 150 mm, Waters) was used for creatinine and d3-creatinine. The mobile phase was composed of a mixture of 0.1% formic acid in water (pH 3.0) and 0.1% formic acid in acetonitrile at the flow rate of 0.2 mL/min. The mass numbers of the molecular and product ions for each compound were as follows: creatinine (114.1→86.1, CE −14 V), and d3-creatinine (117.1→47.1, CE −18 V). Labsolutions software (version 5.89, Shimadzu) was used for data manipulation. The detection limit of creatinine was 10 nM.
Data AnalysisUptake is shown by cell-to-medium ratio, which was obtained by dividing the cellular uptake amount by the concentration of test compound in the uptake medium. Kinetic parameters were estimated by means of nonlinear least-square method using the KaleidaGraph (Synergy Software, Reading, PA, U.S.A.). The Michaelis–Menten constant (Km) for the uptake of xanthine mediated by URAT1 was obtained using the following Michaelis–Menten Eq. 1.
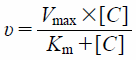 | (1) |
where v, Vmax, Km, and [C] are initial uptake rate, maximal uptake rate, Michaelis constant, and substrate concentration, respectively.
The inhibitory effect of URAT1 inhibitors was expressed as percentage of control, and the inhibitor concentration giving IC50 was obtained by means of the following Eq. 2:
 | (2) |
where [I] is inhibitor concentration.
All data are presented as mean ± standard error of the mean (S.E.M.). Statistical significance was evaluated using Student’s t-test or ANOVA followed by Tukey–Kramer test with a p-value <0.05. Fractional excretion (FE%) of uric acid and xanthine was calculated by dividing their renal clearance by the renal clearance of creatinine.
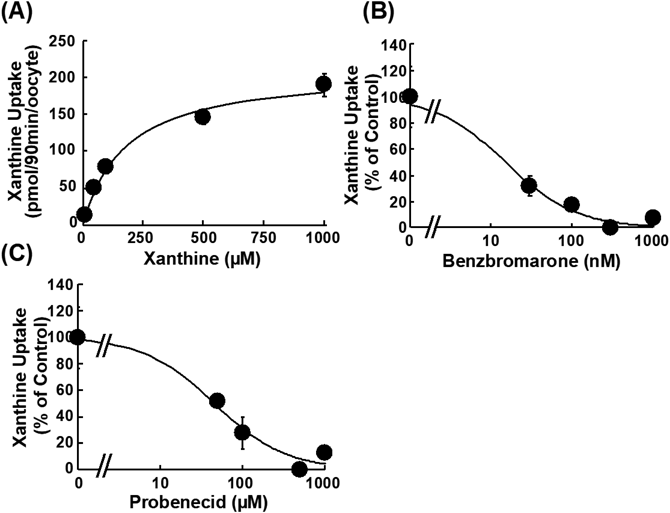
To clarify whether xanthine and hypoxanthine are substrates of URAT1, the uptake of [3H]xanthine and [3H]hypoxanthine by URAT1-expressing and water-injected oocytes was measured for 90 min (Fig. 1A). The uptake of [14C]uric acid and [3H]xanthine by URAT1-expressing oocytes was significantly higher than that by water-injected oocytes, while the uptake of [3H]hypoxanthine by URAT1-expressing oocytes was comparable to that by water-injected oocytes. To confirm intracellular uptake of xanthine, the time course for the uptake of [3H]xanthine by URAT1-expressing oocytes was examined over 120 min. Its uptake increased with time, and the uptakes at 30, 60, 90, and 120 min were significantly higher when compared to those by water-injected oocytes (Fig. 1B). Since the uptake of [3H]xanthine by URAT1-expressing oocytes linearly increased up to 90 min, the following uptake time was set at 90 min. The URAT1-mediated uptake of [3H]xanthine from 20 to 1000 µM was saturable with the estimated Km and Vmax values of 176 ± 25 µM and 212 ± 10 pmol/90 min/oocyte, respectively (Fig. 2A). Moreover, the URAT1 inhibitors benzbromarone and probenecid reduced [3H]xanthine uptake in a concentration-dependent manner with the estimated IC50 values of 15.0 ± 6.3 nM and 44.7 ± 15.2 µM, respectively (Figs. 2B, C). Furthermore, [3H]xanthine uptake by GLUT9-expressing oocytes was significantly higher than that by water-injected oocytes, but less than [14C]uric acid uptake (Fig. 3).

(A) Uptake of [14C]uric acid (10 µM), [3H]xanthine (10 µM), and [3H]hypoxanthine (10 µM) by URAT1-cRNA injected (closed bars) and water injected (open bars) oocytes was measured at 25 °C and pH 7.4. Uptake of [14C]uric acid was performed for 60 min, and that of [3H]xanthine and [3H]hypoxanthine was for 90 min. (B) Time course of [3H]xanthine (10 µM) uptake by URAT1-cRNA injected (closed circles) and water injected (open circles) oocytes was measured at 25 °C and pH 7.4 for 30, 60, 90, and 120 min. Data represents the mean ± standard error of the mean (S.E.M.) from 7–8 oocytes. * indicates significant difference from uptake of each compound by water injected oocytes by Student’s t-test (p < 0.05).
(A) Uptake of [3H]xanthine (10, 50, 100, 500, and 1000 µM) by URAT1-cRNA injected and water injected oocytes was measured at 25 °C and pH 7.4 for 90 min. URAT1-mediated uptake was plotted by subtracting the uptake by water injected oocytes from that by URAT1-cRNA injected oocytes. (B, C) Uptake of [3H]xanthine (10 µM) by URAT1 was measured at 25 °C and pH 7.4 for 60 min in the absence or presence of (B) benzbromarone (0, 30, 100, 300, and 1000 µM) or (C) probenecid (0, 50, 100, 500, and 1000 µM). Data represents the mean ± S.E.M. from 6–8 oocytes.
Uptake of [14C]uric acid (10 µM) and [3H]xanthine (10 µM) by GLUT9-cRNA injected (closed column) and water injected (open column) oocytes was measured at 25 °C and pH 7.4 for 60 min. Data represents the mean ± S.E.M. from 7–8 oocytes, and * indicates significant difference from uptake of each compound by water-injected oocytes by Student’s t-test (p < 0.05).
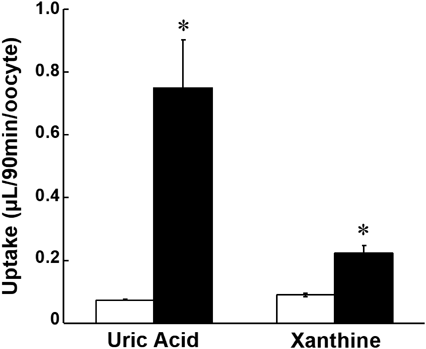
To mimic in vivo tubular reabsorption mediated by URAT1 and GLUT9, the transport of [3H]xanthine by MDCKII cells co-expressing URAT1 and GLUT9 at apical and basolateral membranes, respectively, was examined. Reabsorptive transport of xanthine as well as uric acid from the apical to the basolateral side was significantly higher in cells expressing both transporters than in cells expressing either or none of the transporters (Fig. 4). These results suggested that xanthine is reabsorbed in renal proximal tubular cells via URAT1 and GLUT9, in the same manner as uric acid.
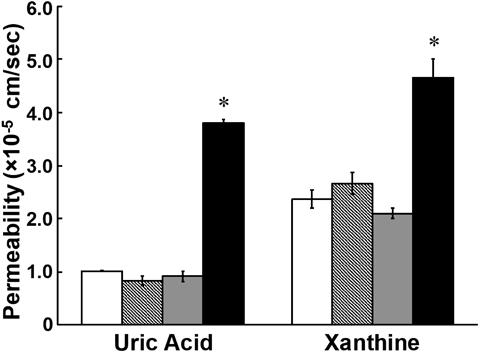
Transcellular transport of [14C]uric acid (20 µM, A) and [3H]xanthine (10 µM, B) across a monolayer of MDCKII cells co-expressing URAT1 and GLUT9 (closed column) in the direction of the apical to basolateral side was compared with that in the MDCKII cells that were mock-transfected (open column), expressing URAT1 alone (hatched column), and expressing GLUT9 alone (gray column). Data represents the mean ± S.E.M. (n = 3). * indicates significant difference from the mock cells by Tukey–Kramer test (p < 0.05).
To investigate whether URAT1 is involved in xanthine reabsorption, urinary excretion clearance of xanthine in rats was investigated by co-administration of topiroxostat and dotinurad. The urinary FE values of uric acid and xanthine in the topiroxostat and dotinurad co-administered group tended to be increased compared with that in the topiroxostat alone administered group (from 38.7 ± 19.6 to 63.8 ± 13.6 for uric acid and 36.8 ± 2.6 to 59.0 ± 15.4 for xanthine), though the change was not statistically significant (Fig. 5).
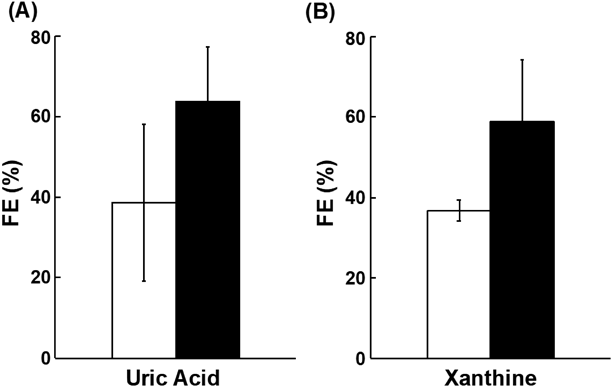
The FE value of uric acid (A) and xanthine (B) was evaluated in rats. Thirty minutes after oral administration of topiroxostat (1 mg/kg) with or without dotinurad (100 mg/kg), blood was taken at 2, 15, and 30 min, and urine was collected over 30 min. The open and closed columns represent mean FE values of each compound in the absence or presence of dotinurad, respectively. Data represents the mean ± S.E.M. (n = 4).
Clinical use of XOR inhibitors for hyperuricemic patients increases plasma concentration of xanthine and hypoxanthine, which leads to an increase of potential uric acid pool. In the present study, we investigated whether urinary excretion of xanthine and hypoxanthine is facilitated by co-administration of URAT1 and XOR inhibitors. In an in vitro uptake study using URAT1-expressing oocytes, xanthine was shown as a substrate of URAT1 (Figs. 1A, B). On the other hand, hypoxanthine was not a substrate of URAT1 (Fig. 1A), suggesting that another mechanism was responsible for hypoxanthine reabsorption. Therefore, the following studies focused on xanthine. To evaluate the potential of URAT1 inhibitors in reducing xanthine reabsorption in vivo, the transport affinity of xanthine for URAT1-mediated uptake was estimated. The Km value of xanthine uptake by URAT1 (176 µM, Fig. 2A) was comparable to that of uric acid (371 µM).12) Furthermore, the IC50 values of benzbromarone (15.0 nM, Fig. 2B) and probenecid (44.7 µM, Fig. 2C) on URAT1-mediated uptake of xanthine were comparable to those for uric acid, 50 nM and 42 µM, respectively.30) These results suggest that URAT1 inhibitors can reduce the reabsorption of xanthine and uric acid when administered to humans. On the other hand, uric acid and xanthine in the urine might compete the uptake into the renal proximal tubule cells each other. However, assuming that urine concentration of uric acid and xanthine is the same as their plasma concentration and the IC50 value is comparable to the Km value, xanthine concentration (0.7–1.6 µM6)) in urine was much lower than the Km value (176 µM), while that of uric acid (287–555 µM6)) is comparable to the Km value (371 µM). These consideration suggests that URAT1-mediated transport of xanthine is partially affected by uric acid. On the other hand, effect of xanthine on reabsorption of uric acid should be negligible.
The reabsorption process requires efflux across the basolateral membrane into the blood after uptake into cells from the renal tubular lumen. For uric acid reabsorption, GLUT9 plays a cooperative role with URAT1.14) As shown in Fig. 3, xanthine is a substrate of GLUT9 as well as URAT1, suggesting that its reabsorption was sequentially mediated by URAT1 and GLUT9 in the same manner as uric acid reabsorption. To confirm the hypothesis, we conducted a transcellular transport study using MDCKII cells that co-express URAT1 and GLUT9, which was established in our previous study.28) As a result, functional co-operation of URAT1 and GLUT9 was experimentally demonstrated by showing higher apical to basolateral transport only in the cells co-expressing URAT1 and GLUT9 (Fig. 4). The involvement of URAT1 in xanthine reabsorption was confirmed by an in vivo study that combined topiroxostat (an XOR inhibitor)31) and dotinurad (a URAT1 inhibitor)25,26) (Fig. 5), which exhibited uricosuric effect in rats.31) The tendency of increase in the FE value of xanthine after administration of dotinurad supported the involvement of URAT1 in xanthine reabsorption. On the other hand, the FE value is determined not only by clearance of xanthine or uric acid, but also by creatinine clearance. In the present study, creatinine clearance was not affected by dotinurad administration (11.5 ± 0.5 mL/min/kg and 10.0 ± 1.1 mL/min with and without dotinurad administration, respectively). On the other hand, excreted amount of xanthine and uric acid into urine was increased by dotinurad treatment, though plasma concentration of xanthine and uric acid was not affected significantly (data not shown). Therefore, the increase of the FE value of xanthine and uric acid was caused by their increased urinary excretion. The reason why their plasma concentrations were not affected by dotinurad may be due to high purine production and uric acid metabolism by uricase, which significantly regulates the plasma concentration of uric acid in rodents, though uricase-mediated metabolism of uric acid is absent in human. Accordingly, it is considered that URAT1 inhibition decreases the plasma concentration of xanthine and uric acid in human by facilitating their urinary excretion. At the same time, the effect of dotinurad on the plasma concentration of hypoxanthine was examined. However, its effect on hypoxanthine could not be evaluated in the present study, since the concentration of hypoxanthine greatly varied with topiroxostat administration (data not shown). Considering that hypoxanthine is not a substrate of URAT1 (Fig. 1A), the FE value of hypoxanthine should not be affected by URAT1 inhibition.
The fact that hypoxanthine is not a substrate of URAT1 is considered beneficial for subjects treated with XOR and URAT1 inhibitors. Previous clinical studies reported the beneficial effect of allopurinol on cardiac event, heart failure, ischemic heart disease, and skeletal muscle disease.32–37) These beneficial effect was accounted for by the increased ATP synthesis from the increased hypoxanthine through the purine salvage pathway under XOR inhibitor treatment.3) Accordingly, a combination of XOR and URAT1 inhibitors could have an advantage in reducing plasma concentration of unfavorable xanthine and uric acid without reducing favorable hypoxanthine.
From the perspective of drug treatment, it might be important to know whether drugs prescribed for hyperuricemia or goat subjects have an inhibitory effect on enzymes and/or transporters affecting purine concentrations other than XOR and URAT1. For example, ATP production from hypoxanthine via the salvage pathway was not affected by topiroxostat, a non-purine-type XOR inhibitor, although the production was inhibited by allopurinol, a xanthine analogue.38) Furthermore, although it is unclear whether xanthine and hypoxanthine are transported by other uric acid transporters, including BCRP (ABCG2), several XOR and URAT1 inhibitors were reported to inhibit BCRP activity,25,39) which has an important role in regulating plasma uric acid concentration by facilitating extra-renal elimination of uric acid into the gut lumen.23,40,41) BCRP was strongly inhibited by benzbromarone and febuxostat at their clinically relevant concentrations, while the effect of allopurinol, dotinurad, probenecid, and topiroxostat on BCRP was less potent.39,42) On the other hand, although hypoxanthine is a substrate of nucleoside transporters ENT1 and ENT2 and nucleobase transporter ENBT1,7–9) its reabsorption mechanism has not been clarified. Clarifying the reabsorption mechanism of hypoxanthine should aid in the consideration of the advantage of XOR inhibitor in the production of ATP from hypoxanthine.
The present study demonstrated that xanthine is reabsorbed in renal proximal tubule via URAT1 and GLUT9, in the same manner as uric acid, and that URAT1 inhibitor facilitated urinary clearance of xanthine. Accordingly, it is expected that the plasma uric acid level could be reduced more effectively by co-treatment with XOR and URAT1 inhibitors to facilitate urinary excretion of xanthine, which was clinically increased by treatment with XOR inhibitor, while hypoxanthine is maintained in the body and used for ATP synthesis via the salvage pathway.
This study was partly supported by Grant-in-Aids for Scientific Research (B), 16H05111, from Japan Society for the Promotion of Science and by Fuji Yakuhin Co., Ltd.
The authors declare no conflict of interest.