2020 Volume 43 Issue 3 Pages 450-457
2020 Volume 43 Issue 3 Pages 450-457
Resveratrol (Res) is a natural active antioxidant that is effective in relieving inflammatory bowel disease (IBD). However, the specific mechanism for its function is unknown. In our study, dextran sodium sulfate (DSS)-induced mouse IBD disease model was constructed. All mice were randomly divided into three groups. The treatment effects of resveratrol on IBD were evaluated by observing the body weight, fecal traits, colon/spleen gross appearance, tissue hematoxylin–eosin (H&E)/immunohistochemistry (IHC) and inflammatory factors. The expression of small ubiquitin-like modifier protein 1 (SUMO1) and its Wnt/β-catenin pathway-related genes was analyzed by IHC, Western blot, Real-time PCR (RT-PCR) and Immunofluorescence. The outcome indicated that resveratrol treatment significantly relieved the symptoms of IBD. The expression level of anti-inflammatory cytokines was increased while that of pro-inflammatory cytokines was decreased in both colon and spleen tissues of resveratrol-treated mice. SUMO1 expression and Wnt/β-catenin pathway were suppressed in colon and spleen tissues of IBD mice treated with resveratrol. In addition, we provided evidence that resveratrol inhibited SUMO1 and β-catenin expression and their nuclear localization in human colonic epithelial cell line (FHC). Moreover, we found that SUMO1 and β-catenin had higher expression levels in colorectal cancer patients than in health and colitis patients. In conclusions, resveratrol alleviates DSS-induced IBD by modulating SUMO1 through Wnt/β-catenin pathway.
Inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), is attributable to the complex interactions between genetic, immune, microbial and environmental factors.1) Collectively, these factors lead to an increase in inflammatory mediators, forming a cascade effect that causes uncontrolled inflammatory responses leading to irreversible tissue damage. Recent epidemiological data show that the prevalence and incidence of IBD in China have risen sharply over the past two decades.2) It is generally documented that, IBD increases the risk of colitis-associated colorectal cancer (CAC).3) Although several methods have been used to treat IBD, the effects and outcome of these treatment methods are still largely unsatisfactory.4) Therefore, there is an urgent need to find alternative methods of treating IBD in the clinic.
Resveratrol is a natural polyphenolic compound that is enriched in mulberries, Polygonum cuspidatum, grapes, and red wine.5) Previous research has demonstrated that resveratrol has anti-inflammatory and anti-oxidant activity.6) In vitro and in vivo studies have confirmed that resveratrol can ameliorate IBD by reducing mucosal inflammation,7,8) however, the specific mechanism by which resveratrol works is currently unclear.
Small ubiquitin-like modifier protein (SUMO) is a highly conserved small protein that is widely present in eukaryotes and its involvement in SUMOylation is a vital post-translational modification of proteins in cells. SUMO1 is a member of the SUMO gene family and its modification regulates many protein functions such as P53, proliferating cell nuclear antigen (PCNA) and telomerase.9–11) In addition, SUMO1 is also involved in the regulation of signaling pathways such as nuclear factor-kappaB (NF-κB), Wnt/β-catenin, transforming growth factor-β (TGF-β) and c-Jun N-terminal kinase (JNK).12,13) Based on the characteristics of SUMO1, it plays an important role in many diseases such as inflammation-related diseases, tumors, and kidney diseases.14–16)
In our study, we researched the role of resveratrol in inhibiting the expression of SUMO1 and the Wnt/β-catenin pathway in dextran sodium sulfate (DSS)-induced IBD. Furthermore, the expression of SUMO1 in different processes of human colorectal cancer development was examined.
Human normal intestinal FHC cells were cultured in RPMI 1640 media containing 10% fetal bovine serum (FBS) at 37°C, 5% CO2. Resveratrol (Sigma, U.S.A.) formulated into a 20 µmol/mL stock solution in dimethyl sulfoxide (DMSO) and stored at −20°C. The stock solution was diluted with the medium to the desired concentration for use. The concentration used in this study is 20 µmol/L.
Animal ModelBALB/c mice (6–8 weeks old, male) were purchased from the Laboratory Animal Research Center of Jiangsu University (Jiangsu, China). All experimental procedures follow the guidelines of the Animal Care and Nursing Committee of Jiangsu University.
We employed the random principle to divide mice into three groups (n = 6/group): control group (Normal), untreated colitis group (DSS), resveratrol-treated colitis group (DSS + Res). The control group received regular drinking water throughout the study. In the other two groups, colitis was induced by providing 3% DSS (MP Biomedicals, U.S.A.) drinking water for 10 d. During this period, the mice in the DSS + Res group were given resveratrol 100 mg/(kg.d) daily. Body weight, the stool consistency and bleeding in mice were monitored daily. In addition, the DAI score (Table 1) was used to describe the severity of colitis. All mice were sacrificed on day 11 and their colon and spleen tissues were collected for further study.
| Score | Weight loss (%) | Stool consistency | Stool consistency |
|---|---|---|---|
| 0 | None | Normal | Normal |
| 1 | 1–5 | ± | |
| 2 | 5–10 | Loose stool | + |
| 3 | 10–15 | ++ | |
| 4 | More than 15 | Watery diarrhea | +++ |
DAI = (weight loss + stool consistency + rectal bleeding)/3.
Paraffin-embedded mice colon and spleen tissue (4 µm thick) was fixed with formalin and stained with hematoxylin–eosin (H&E) or dewaxed for immunohistochemistry. 3% hydrogen peroxide was used for 30 min to inhibit endogenous peroxidase activity and antigen retrieval was achieved by boiling in citrate buffer for 30 min. Sections were then blocked with 5% bovine serum albumin (BSA) and incubated with primary antibody such as SUMO1 (1 : 250, Abcam, U.K.), PCNA (1 : 200, Cell Signaling Technology, U.S.A.), β-catenin (1 : 100, Cell Signaling Technology) at 4°C overnight. Finally, sections were visualized using diaminobenzidine (DAB) (Bosterbio, U.S.A.) substrate and counterstained with hematoxylin for microscopy.
ImmunofluorescenceFHC cells were fixed with 4% paraformaldehyde for at least 30 min, ruptured with 0.1% Triton-X-100 for 20 min, and blocked with 5% BSA for 30 min, and then incubated with anti-SUMO1 (1 : 250, Abcam) and anti-β-catenin (1 : 100, Cell Signaling Technology) at 4°C overnight, followed by incubation with diluted secondary antibody at 37°C for 1h. The nucleus was counterstained with hoechst33342 (1 : 300, Sigma) for 10 min at room temperature. Images were taken by confocal microscope (Nikon, Japan).
Real-Time (RT) PCRTotal RNA in the colon and spleen tissues was extracted by TRIzol Reagent (Gibco, U.S.A.), and cDNA was synthesized using the HiScript 1st Strand cDNA Synthesis Kit (Vazyme Biotech, China). The mRNA expression was also analyzed using RT-PCR and β-actin was used as an internal control. The sequences of primer pairs are listed in Table 2.
| Gene | Primer sequence (5′→3′) | Annealing temp (°C) | Amplicon size (bp) |
|---|---|---|---|
| IL-6 | FOR: AAGTCCGGAGAGGAGACTTC | 60 | 487 |
| REV: TGGATGGTCTTGGTCCTTAG | |||
| IL-8 | FOR: CATCTTCGCTGTCGTCCTT | 60 | 326 |
| REV: AGAGGGTAGTAGAGGTGTTTGC | |||
| IL-10 | FOR: CCTGGCTCAGCACTGCTATG | 58 | 151 |
| REV: TCACCTGGCTGAAGGCAGTC | |||
| IL-1β | FOR: AGCTTCAGGCAGGCAGTATC | 60 | 215 |
| REV: TCATCTCGGAGCCTGTAGTG | |||
| TNF-α | FOR: AACTCCAGGCGGTGCCTATG | 60 | 242 |
| REV: TCCAGCTGCTCCTCCACTTG | |||
| SUMO1 | FOR: TGCTGTAGAGAAGGGACGGA | 58 | 108 |
| REV: TGGTCAGACATGGTGACGTG | |||
| SENP1 | FOR: AGCCTGTAGCTCGCTCTTTG | 58 | 273 |
| REV: GCCGTGGTTCACTAAGGTCA | |||
| β-Actin | FOR: GACCTGTACGCCAACACAGT | 59 | 129 |
| REV: CTCAGGAGGAGCAATGATCT |
Radio-Immunoprecipitation Assay (RIPA) lysis buffer was added to colon tissue, spleen tissue and FHC cells and the protein concentration was measured by Bicinchoninic acid (BCA) method. A total of 200 µg of protein sample was separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The separated protein was transferred to a polyvinylidene difluoride (PVDF) membrane that had been activated with methanol and blocked with 5% skim milk for at least 1h at room temperature. Then incubation with primary antibody at 4°Covernight, the antibodies incubated mainly include anti-SUMO1 (1 : 1000, Abcam), anti-β-catenin (D10A8) (1 : 1000, Cell Signaling Technology), anti-Wnt5a (1 : 1000, Cell Signaling Technology), and anti-β-actin (1 : 800, Santa Cruz Biotechnology, U.S.A.). The second day, the blots were incubated with the goat anti-rabbit immunoglobulin G (IgG) for 1 h at 37°C, and then were visualized by chemiluminescence (Millipore, U.S.A.), and imaged using imaging software (GE Healthcare, Life Sciences, U.S.A.).
Statistics AnalysisAll experiments follow the principle of repetitiveness and data expressed as mean ± standard deviation (S.D.). One way ANOVA was performed for comparisons among three groups. The significance of the differences was assessed by GraphPad Prime5 software with p < 0.05 considered as significant.
| Health control | Ulcerative colitis | Colorectal cancer | |
|---|---|---|---|
| Total subject; n | 10 | 30 | 34 |
| Man; n | 5 | 19 | 20 |
| Woman; n | 5 | 11 | 14 |
| Age, years, mean (rang) | 59 (55–76) | 61 (52–74) | 64 (55–78) |
| Colitis stage | |||
| Mild | — | 13 | — |
| Moderate | — | 9 | — |
| Severe | — | 8 | — |
| Tumor stage | |||
| I | — | — | 2 |
| II | — | — | 18 |
| III | — | — | 10 |
| IV | — | — | 4 |
To investigate the role of resveratrol in DSS-induced colitis, we first established a stable model of inflammatory bowel disease induced by 3% DSS. We then split this group into two and treated one group with resveratrol whilst the other group was left untreated. Upon examination, we noticed that mice treated with resveratrol had longer colon length and smaller spleen as compared to the untreated colitis group (Figs. 1A–D). No significant differences in weight loss were observed between the groups (Fig. 1E). However, the mice in DSS group showed early blood-stains in the stool, which can be seen from the DAI curve (Fig. 1F). As indicated by the H&E staining (Fig. 1G), the colon and spleen structures of colitis mice were severely disrupted, while DSS + Res group did not show significant structural disturbances. Moreover, immunohistochemical analysis showed that PCNA was higher in colon and spleen tissues of mice treated with resveratrol than in those without treatment (Fig. 1H). These observations indicate that, resveratrol administration significantly inhibited DSS-induced symptoms of colitis in mice.

A. The colon morphology of mice among three groups. B. The spleen morphology of mice among three groups. C. Colon length statistical analysis among three groups. D. Spleen weight statistical analysis among three groups. E. The body weight of mice among three groups. F. The clinic disease activity index (DAI) of mice. G. H&E staining results of colon and spleen tissues in mice (100×, scale bar = 100 µm). H. IHC to detect the expression level of PCNA in both colon and spleen tissues of mice (100×, scale bar = 100 µm). (Color figure can be accessed in the online version.)
The measurement of the expression of pro-inflammatory cytokines in colon and spleen tissues was carried out using RT-PCR. Information in the three groups about the expression levels of anti-inflammatory cytokines of colon tissues indicated that, the expression of pro-inflammatory cytokines significantly reduced after resveratrol treatment. These cytokines include; tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-8 and IL-6 (Figs. 2A–D). Instead, anti-inflammatory cytokines IL-10, increased dramatically in DSS + Res group than those in the DSS group (Fig. 2E). Similar results were confirmed in the spleen tissues from the DSS group and the DSS + Res group (Fig. 3).

A–E. Gene expression levels of pro-inflammatory and anti-inflammatory factors, shown in the order of IL6, IL8, TNF-α, IL-1β and IL10 in the colon tissues. Bars represent the means ± S.D. ** p < 0.01; *** p < 0.001.
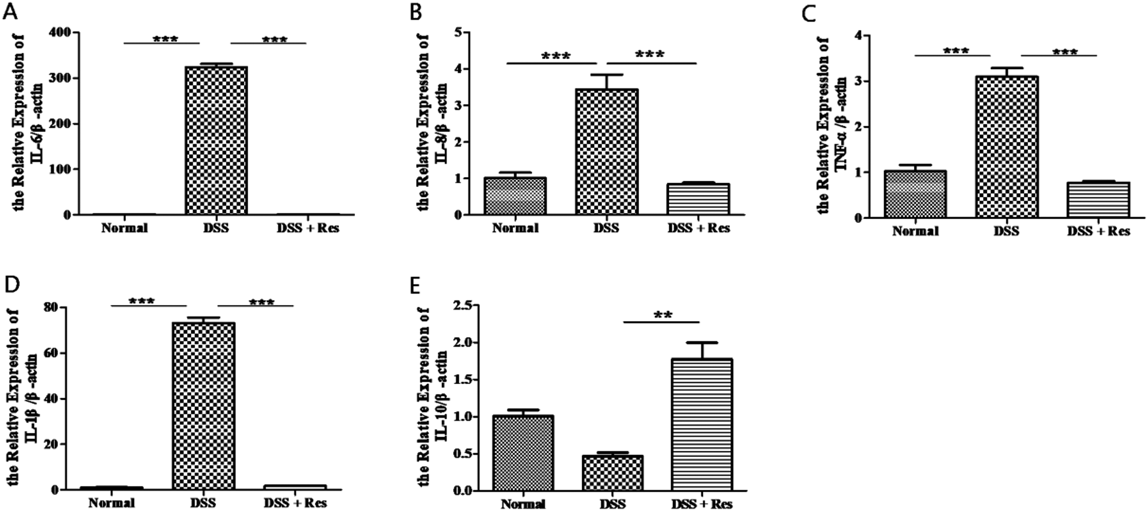
A–E. Gene expression levels of pro-inflammatory and anti-inflammatory factors, shown in the order of IL6, IL8, TNF-α, IL-1β and IL10 in the spleen tissues. Bars represent the means ± S.D. ** p < 0.01; *** p < 0.001.
In order to determine whether resveratrol attenuate the DSS-induced colitis by regulating SUMO1, we studied the expression of SUMO1 in colon and spleen tissues among the three groups. Expression of SUMO1 increased in the colon tissues of DSS group while it decreased for the DSS + Res group. Similar results were found in spleen tissues (Figs. 4A, D, G–J). SENP1, a nuclear SUMO protease, which mediates DeSUMOylation has been shown to be involved in the development of many diseases.17) Upon measuring the expression of SENP1 gene, it was clearly evident that both colon and spleen tissues showed an increment in the expression of SENP1 gene in DSS + Res group (Figs. 4B, E). In addition, SUMO-conjugating enzyme Ubc9 was also down-regulated in the DSS + Res group (Figs. 4C, F). Immunohistochemistry also reflected that, DSS group had more SUMO1+ cells in both colon and spleen tissues than DSS + Res group (Fig. 4K).

A, B, C. The gene expression of SUMO1, SENP1 and UBC9 in colon tissues was detected by RT-PCR. D, E, F. The gene expression of SUMO1, SENP1 and UBC9 in spleen tissues was detected by RT-PCR. G. The Western blot result of SUMO1 in the colon tissues. H. Grayscale analysis result of SUMO1 in the colon tissues. I. The Western blot result of SUMO1 in the spleen tissues. J. Grayscale analysis result of SUMO1 in the spleen tissues. K. IHC to detect the expression level of SUMO1 in the colon and spleen tissues (100×, scale bar = 100 µm). Bars represent the means ± S.D. * p < 0.05; ** p < 0.01; *** p < 0.001. (Color figure can be accessed in the online version.)
To verify whether SUMO1 acts through the Wnt/β-catenin pathway to relieve colitis, we detected the expression levels of Wnt/β-catenin-related proteins in colon tissues. A higher expression of Wnt5a and β-catenin protein appeared in DSS group compare to normal group. As a consequence of the treatment administered, DSS + Res group reversed the changes caused by the induced-colitis (Figs. 5A–C). Moreover, spleen tissues also showed similar results (Figs. 5D–F). Further confirmation with immunohistochemistry reflected that DSS group had more β-catenin+ cells in the colon and spleen tissues than DSS + Res group (Fig. 5G).
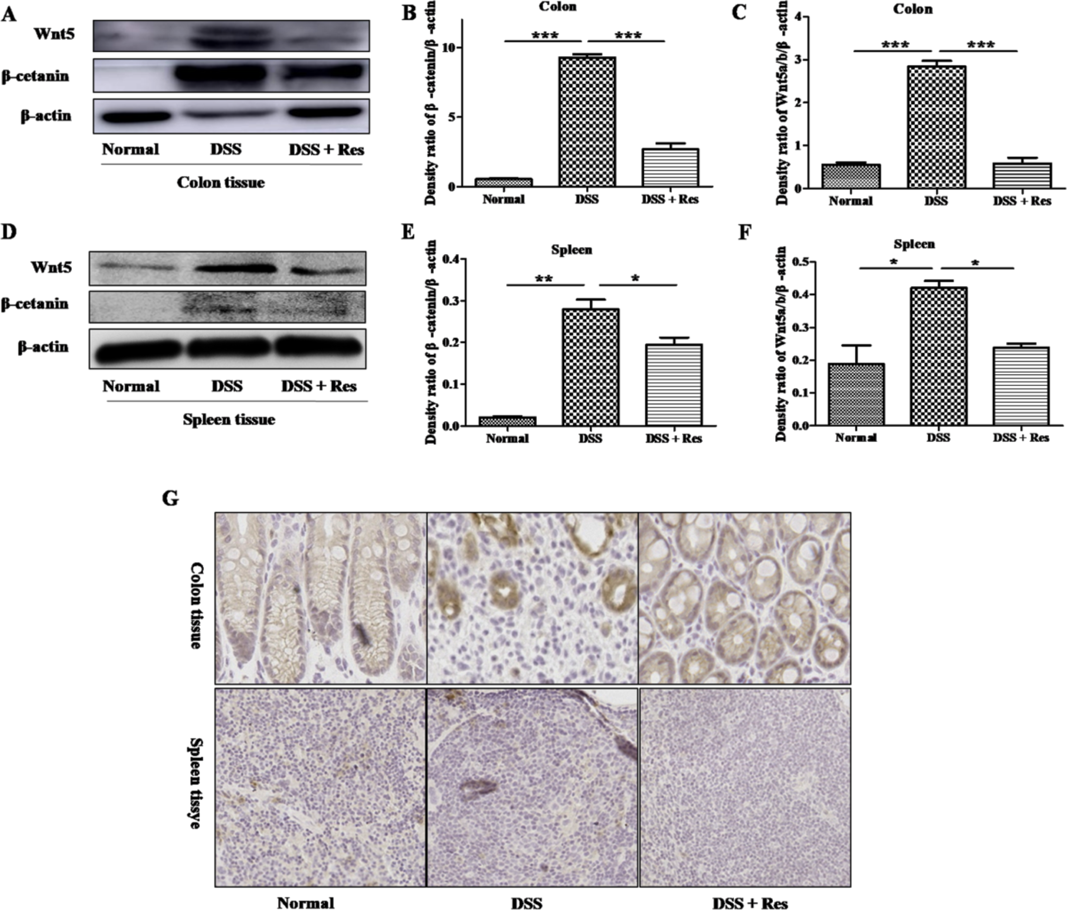
A. The Western blot results of Wnt5a and β-catenin in the colon tissues. B, C. Grayscale analysis results of β-catenin and Wnt5a in the colon tissues. D. The Western blot results of Wnt5a and β-catenin in the spleen tissues. E, F. Grayscale analysis results of β-catenin and Wnt5a in the spleen tissues. G. IHC to detect the expression level of β-catenin in both colon and spleen tissues (100×, scale bar = 100 µm). Bars represent the means ± S.D. * p < 0.05; ** p < 0.01; *** p < 0.001. (Color figure can be accessed in the online version.)
Intestinal epithelial cells have been indicated to play a key role in the development of IBD.18) Therefore, we examined the expression of SUMO1 and β-catenin in FHC cells to access the quantitative manifestation of these molecules in the normal foetal colonic cells. We observed that SUMO1 expression in these cells decreased after treatment with resveratrol. Moreover, DSS processing resulted in an obvious increase in β-catenin and Wnt5a expression, whereas resveratrol treatment resulted in a substantial decrease (Figs. 6A–D). In addition, immunofluorescence analysis showed that DSS treatment promoted the entry of SUMO1 and β-catenin into the nucleus of these cells. However, after resveratrol treatment, SUMO1 and β-catenin were appreciably reduced in the nucleus (Figs. 6E, F).

A. The Western blot results of SUMO1, β-catenin and Wnt5a in the FHC cells. B. Grayscale analysis results of Wnt5a. C. Grayscale analysis results of β-catenin. D. Grayscale analysis results of SUMO1. E. Immunofluorescence staining of SUMO1 in the FHC cells (40×, scale bar = 100 µm). F. Immunofluorescence staining of β-catenin in the FHC cells (40×, scale bar = 100 µm). Bars represent the means ± S.D. * p < 0.05; ** p < 0.01; *** p < 0.001. (Color figure can be accessed in the online version.)
Furthermore, we examined the expression of SUMO1 and β-catenin in colon tissues from healthy controls, ulcerative colitis patients and colorectal cancer patients by using immunohistochemistry. However, we found that there were more SUMO1+ cells and β-catenin+ cells in the colon tissues of colorectal cancer patients than in healthy controls and ulcerative colitis patients (Fig. 7).

A. IHC of SUMO1 expression from healthy control, ulcerative colitis patients and colorectal cancer patients (100×, scale bar = 100 µm). B. IHC of β-catenin expression from healthy control, ulcerative colitis patients and colorectal cancer patients (100×, scale bar = 100 µm). (Color figure can be accessed in the online version.)
IBD is a kind of chronic inflammatory diseases. Although the cause of IBD remains unclear, it has been reported that intestinal mucosal barrier dysfunction, abnormal immune response, genetic factors and environmental degradation may lead to the pathogenesis.19–21) In addition, the dysregulation between pro-inflammatory cytokines and anti-inflammatory cytokines further aggravates mucosal inflammation in IBD.22) Therefore, inhibition of intestinal inflammation plays a vital role in the treatment of IBD.
Resveratrol has an anti-inflammatory effect that relieves symptoms of inflammatory diseases such as IBD.23) Martín et al.24) demonstrated that resveratrol can alleviate intestinal mucosal injury by decreasing the recruitment of neutrophils and the secretion of TNF-α. Our results are in accord with this earlier findings suggesting that resveratrol inhibits the expression of pro-inflammatory cytokines and relieves colitis, although the mechanisms involved largely remain elusive. In this study, we demonstrated that SUMO1 plays an important role in the onset and progression of IBD hence the remission of IBD by resveratrol crucially impart the expression of SUMO1. From our results, resveratrol effectively inhibited the expression of SUMO1 in both colon and spleen tissues and as well inhibit the nuclear translocation of SUMO1 in FHC cells.
Numerous studies have shown that SUMO1 is covalently bound to more than 100 cellular proteins25) and that, SUMO1 mediated SUMOylation pathway are implicated in many aspects of controlling cell physiology, including cell cycle regulation, transcription and nuclear transport.26) One of the obvious functions of SUMO1 is its ability to bind to β-catenin and enhance transcriptional activity.13) Moreover, the activation of β-catenin is closely related to the malignant transformation of colon and upper gastrointestinal adenocarcinoma.27,28)
Although Wnt signaling is not the mainstream inflammatory pathway, there is increasing evidence that it can be used as a driver of injury repair.29) Nevertheless, the Wnt/β-catenin pathway activation is a known characteristic of chronic IBD in animal models.30) Larrosa et al.31) found in the DSS-induced rat colitis model that the Wnt signaling gene was significantly regulated by resveratrol treatment.
On this background, we hypothesized that resveratrol can attenuate inflammation by inhibiting SUMO1 expression and nuclear translocation, which in turn will affects the activity of Wnt/β-catenin pathway. Our study showed that resveratrol effectively inhibited Wnt/β-catenin pathway. Further in vitro experimental analysis indicated that, resveratrol does not only inhibit the expression of β-catenin, but also inhibits their nuclear translocation. This is a new conceptual model in which resveratrol down-regulates SUMO1, resulting in the inhibition of Wnt5a expression and β-catenin activation, and consequently inhibiting SUMO1 and β-catenin from entering the nucleus, finally impeding the Wnt/β-catenin pathway to cause remission to achieve inflammation-reducing effects (Fig. 8) .

(Color figure can be accessed in the online version.)
In addition, we examined the expression of human tissues SUMO1 and β-catenin and found that expressions of these molecules increase as the disease progresses from mere inflammation to a carcinoma. This provides new clinical indicators for clinical judgment or resveratrol treatment.
In conclusion, resveratrol alleviate IBD by inhibiting the expression of SUMO1 and reducing inflammatory responses, which points a new direction for the clinical treatment of IBD.
This study is supported by the National Natural Science Foundation of China (Grant No. 81670502), Tai-cang Science and Technology Planning Project (Grant no.TC2018JCYL13), the Scientific Research Foundation of Jiangsu University (Grant No. FCJJ2015023), the opening project of the Key Laboratory of Embryo Molecular Biology, Ministry of Health of China, and Shanghai Key Laboratory of Embryo and Reproduction Engineering (Grant No. KF201601), Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions, and College student practice innovation training program, Orientation projects, Jiangsu Province (Grant No. 201910299233Y).
The authors declare no conflict of interest.