2020 Volume 43 Issue 3 Pages 375-383
2020 Volume 43 Issue 3 Pages 375-383
Recent progress in the fields of tissue engineering, micro-electro mechanical systems, and materials science have greatly improved cell culture systems, which were traditionally performed in a static two-dimensional manner. This progress has led to a number of new cell culture concepts represented by organ-on-a-chip, three dimensional (3D)-tissues, and microphysiological systems, among others. In this review, these culture models are categorized as reconstituted human organ models, which recapitulate human organ-like structure, function, and responses with physiological relevance. In addition, we also describe the expectations of reconstituted organ models from the viewpoint of a pharmaceutical company based on recent concerns expressed in drug discovery and development. These models can be used to assess the pharmacokinetics, safety and efficacy of new molecular entities (NMEs) prior to clinical trials. They can also be used to conduct mechanistic investigations of events that arise due to administration of NMEs in humans. In addition, monitoring biomarkers of organ function in these models will aid in the translation of their changes in humans. As the majority of reconstituted human organ models show improved functional characteristics and long-term maintenance in culture, they are valuable for modeling human events. An example is described using the three-dimensional bioprinted human liver tissue model in this article. Implementation of reconstituted human organ models in drug discovery and development can be accelerated by encouraging collaboration between developers and users. Such efforts will provide significant benefits for delivering new and improved medicines to patients.
Recent progress in cell culture technology has led to increasing focus on ways to use this emerging technology in the pharmaceutical industry for drug discovery and development. Indeed, several buzzwords have arisen in the field, including three dimensional (3D) culture, spheroid culture, microphysiological system (MPS), organ-on-a-chip, body-on-a-chip, organoid, and organoid-on-a-chip. “Organs-on-chips” was among the top 10 emerging technologies in 2016 in the World Economic Forum,1) and “organoid” was the Method of the Year in 2017 according to a Nature Methods article.2) Many review articles3–7) have aimed to improve understanding of the technology, as well as its principles, research history, and potential in the life sciences. While these are valuable reviews, few articles have reviewed the practical use of such models in drug discovery and development.
The present review aims to summarize the application of these cell culture models in drug discovery and development from the perspective of a pharmaceutical company. For the purpose of this review, we refer to these advanced cell culture models as reconstituted human organ models and compare them with other human organ models such as isolated organs and human cells/organs in chimeric animals. This will help to provide a realistic understanding of these emerging technologies and provide new insight to cell culture models. We will also describe recent issues encountered in drug discovery and development, human organ models as one solution to these issues, a case study on the use of a reconstituted human organ model of the liver, and future challenges.
Traditionally, drug discovery encompasses the provision of industrial resources dedicated to the creation and identification of a candidate or candidate groups of new molecular entities (NMEs) for clinical trial. Assessments of pharmacokinetics, safety, and efficacy in humans are keys to this stage. Some of these study results may be used, in addition to other study results, for future applications to regulatory bodies for a first-in-man study. The clinical development plan is also strategized based on these pre-clinical study results and appropriately updated in accordance with study progress and changes in external circumstances such as new scientific findings and the status of competitors. While the successful initiation of a first-in-man study tends to be considered a goal for pre-clinical research, it may only be a starting point for clinical trials. This discrepancy may be due to differences in the definition of “outcome” between drug discovery and development stages8) despite sharing the same goal, namely, delivering new and improved medicines to patients. In addition to this conceptual gap, another gap exists between pre-clinical research and clinical trials: pre-clinical research is conducted to select NMEs for clinical trials that will show benefits for patients without actually exposing humans to these compounds. Indeed, clinical trials often fail, confounding expectations that were based on findings from pre-clinical research. This is one reason, and perhaps the main reason, for the low productivity in drug development in terms of cost, time and labor. Major reasons for the low success rate for progression of compounds to higher trial phases include lack of efficacy and unexpected safety issues.9) Several potential solutions to this issue have been described. Morgan et al.10) reported that a fundamental understanding of the relationship between an NME’s pharmacokinetics and pharmacodynamics is important for the translation of pre-clinical findings to expected outcomes in humans. This same report emphasized that confidence in target exposure and pharmacological action are key to improving candidate survival in Phase II trials. In addition, a case report from AstraZeneca11) showed that the success rate of clinical trials improved after incorporating the concept of a five-dimensional framework into their decision-making, which consisted of focusing on the right target, tissue, safety, patient, and commercial potential. This same report suggested that new cell culture models, such as 3D liver microtissues, organoids, and microphysiological systems, would improve the translation of pre-clinical findings to clinical outcomes.
Pre-clinical research aims to gain the best understanding of human responses to candidate NMEs before a first-in-human study, and accordingly necessitates the use of human samples. Indeed, human liver microsomes and hepatocytes are often used to evaluate drug disposition properties in humans.12) These approaches are thought to have contributed to the reduction in the attrition rate of Phase I studies in the 1990s, a period when failure was widely ascribed to inadequate pharmacokinetic profiles and bioavailability of drugs.13) Similarly, it is reasonable to expect that human samples will also benefit assessment of the safety and pharmacological profile of NME candidates in pre-clinical research. To date, human specimens for such assessments have been primarily used in a “static” manner; for example, human blood, urine, fixed tissue sections, biopsy specimens, and isolated organs from post-mortem donors have been used for mRNA and protein expression analysis, -omics approaches, and biomarker exploration and monitoring. Additional “dynamic” functional analyses using human specimens that correlate with human responses are strongly desired to improve the quality of NME assessment in pre-clinical research. For this purpose, “viable” human specimens are highly sought after.
Figure 1 introduces the concept of the human organ model for drug discovery and development, allowing viable functional evaluation of human organ responses. Human organ models can be used as a complement or substitute for animal models. In particular, they can provide valuable data in cases where a suitable animal model is unavailable. Table 1 summarizes the classifications of human organ models applicable to functional analysis: isolated organs, cells/organs in chimeric animals, and reconstituted organs. Among isolated organs, liquid specimens such as blood and sperm can be used for coagulation and motility studies, respectively. In contrast, solid organs such as liver and kidney excised from patients or post-mortem donors are sometimes reserved for pre-clinical research. Tissue slices from these organs can be used to investigate metabolic function in the liver14) and transporter uptake function in the kidney.15) Although these strategies are one solution to the use of human tissues for functional analysis, tissue viability cannot be maintained, prohibiting long-term study. Chimeric animals with humanized tissue are a unique type of human organ model. Human-derived cells grafted into immunocompromised animals receive blood flow from the host animal, allowing the practical administration of chemicals to these animals. These animals live for a period of weeks, allowing long-term study.16,17) While an increasing variety of organs is expected to be available for use in the future, it should be noted that these chimeric animals may exhibit irregular immune modulation because their immune systems have been modified to accept human-derived cells.

Human organ models are expected to be used to assess the pharmacokinetics, safety, and efficacy profiles of new molecular entities (NMEs) before clinical trials as a complementary method to animal models. In addition, human organ models can be used to reflect human responses to NMEs observed in clinical trials, enabling mechanistic investigations of these outcomes. (Color figure can be accessed in the online version.)
| Model | Example | Advantage | Drawback |
|---|---|---|---|
| Isolated organs | Blood, sperm | Preserved complexity of cell type and structure | Rapid loss of functionality |
| Tissue slice such as liver, kidney | Limited opportunity for access | ||
| Cells/organs in chimeric animals | Human liver chimeric mice | Connected to blood circulation | Limited variety of organs to date |
| Human tumor xenograft animals | Immunocompromised condition | ||
| Reconstituted organs | Organ-on-a-chip, microphysiological system, 3D-culture, organoids | Long-term culture with high functionality once established | Better cell source required |
| Characterization needed |
Reconstituted human organ models are a new concept and may be an option among human organ models (Table 1). To date, numerous new terms related to cell culture systems have arisen, including organ-on-a-chip, body-on-a-chip, and microphysiological systems, among others. However, as is the nature of emerging technologies, it is difficult to definitively define these terms. Instead, applying the concept of the reconstituted human organ model to these terms based on their common perceptions will enable their advantages to be well organized and emphasized. Reconstituted human organ models are systems that can depict human organ responses ex vivo. They partially or mostly mimic the circumstances of the original organ beyond the static 2D, single-cell-type approach. Improvements to the environment of cells may include placing cells in 3D systems where they neighbor other cell types that are present in the organ in vivo, and applying physical drivers to the systems. As a result, the physiological relevance of reconstituted organ models is expected to be greater than that of traditional cell culture models, and they will continue to be updated with further improvements. The definitions described above will aid in identifying practical uses for reconstituted human organ models, and three examples of which are detailed below.
First, these models can be used when researchers need to evaluate human responses to a certain event that is observed in animal models. In this case, they may start by focusing on a key event observed in animals, and try to represent the event in reconstituted human organ models. Because current reconstituted human organ models may not perfectly mimic human organs, researchers should focus on key events observed in animals. For example, if the distribution of a specific compound/biologic in the brain is improved in animals following certain modifications, the human blood–brain barrier model can be used to assess the compound/biologic’s pharmacokinetics profile in humans. Additionally, if metabolites of an administered compound can be recovered from feces in an animal study, the human hepatobiliary model (i.e., sandwich-cultured hepatocyte model) can be used to assess drug disposition in humans. An advantage of reconstituted organ models is that a variety of experimental settings, such as those for time–course profiling, concentration dependency, and co-incubation scenarios like drug-drug interactions, are applicable compared to other organ models due to their relatively higher throughput.
Second, in another situation that arises in drug discovery and development, researchers may want to investigate the details of events that are observed in clinical trials. In this case, it is more reasonable to use human organ models rather than animal models because the event was observed in humans. For example, if organ-specific toxicity is observed for an NME in clinical trials, such as hepatotoxicity, human liver models can be used to mimic such events. Once hepatic damage is modeled in an ex vivo manner, mechanistic investigation can be conducted on these models. These studies are important for forecasting potential risks to conducting further clinical trials on the NME and for nominating other NMEs for clinical trials.
Third, reconstituted human organ models may be useful for monitoring biomarkers, which are measurable parameters in clinical trials. For example, aspartate aminotransferase and alanine aminotransferase are major enzymes used to evaluate liver function in humans. Ogihara et al.18) reported that a 3D spheroid model of the liver could be used to detect these enzymes in culture media. They also investigated their levels after treatment with hepatotoxicants. In another example, Maass et al.19) successfully detected kidney-injury molecule 1 (KIM-1), a biomarker for monitoring proximal tubule damage, in the effluent of a microphysiological model of the human kidney proximal tubule. They also reported changes in KIM-1 levels during co-treatment with nephrotoxic chemicals. These reports suggest that reconstituted human organ models can be a powerful tool for translationally evaluating human organ-specific events in detail.
Among the variety of human organs, the liver appears to be the most actively used for human organ models. Two reasons may explain this. The first is that various cell types can be practically collected from the human liver, such as hepatocytes, stellate cells, and Kupffer cells, which enables the development of more complicated co-culture models that are expected to retain liver functionality. The second reason is the fact that the liver is the organ in which safety issues most frequently arise, leading to the termination of clinical trials and even withdrawal of drugs from the market.20) By the 1990s, hepatocytes had already been cultured beyond the traditional 2D-culture manner, including in sandwich-culture with extracellular matrices and spheroid culture.
In 2016, Nguyen et al.21) reported the original bioprinted 3D human liver model and the investigation of its characteristics and response to trovafloxacin, which is known to cause hepatotoxicity in humans. The 3D-bioprinted liver tissue model consisted of human primary hepatocytes, stellate cells, and umbilical vein endothelial cells, and was generated using a 3D-bioprinter by a microextrusion method. The model showed positive immunostaining for albumin, desmin, and CD31, markers of hepatocytes, stellate cells, and endothelial cells, respectively. It also showed mRNA expression of CYP 1A2, CYP2B6, CYP2C9, CYP2D6, and CYP3A4 up to day 28 post-printing. Further, rifampicin treatment induced CYP3A4 expression in the model. In addition, trovafloxacin exhibited hepatotoxic responses such as reduced levels of tissue albumin and ATP in a concentration-dependent manner. This reduction was less sensitive in standard 2D-cultured hepatocytes. Histological analysis also revealed loss of cellular adhesion and necrosis following trovafloxacin treatment. Importantly, trovafloxacin causes hepatotoxicity in humans,22) while trovafloxacin alone does not cause hepatotoxicity in rats. Meanwhile, co-treatment with lipopolysaccharide (LPS) is required to detect hepatotoxicity of trovafloxacin.23) These results suggest that the 3D-bioprinted liver tissue model is advantageous for detecting the hepatotoxic potential of compounds in humans, while animal studies require some artificial modifications for detecting hepatotoxicity.
We investigated the effect of acetaminophen (APAP) on the 3D-bioprinted human liver tissue model24) reported by Nguyen et al. Figure 2 shows the tissue ATP level after treatment of the model with APAP for up to 28 d. After 1 d, treatment with the highest concentration of APAP (30 mM) had reduced the ATP level to 27.9% of the untreated control level (Fig. 2A). Prolonged APAP treatment duration decreased the APAP concentration available to affect the ATP level. For example, 28 d of APAP treatment at 0.3 mM reduced ATP levels to approximately 50% (Fig. 2D), while 1 d of treatment at this APAP concentration had no effect on ATP levels. The half-toxic concentration (TC50) value decreased over the APAP treatment period (Fig. 2E). We also studied the effect of short-term treatment of APAP and the washout effect (Fig. 3). Twenty-four hours of APAP treatment at 30 mM significantly reduced ATP levels, while 6 h of treatment had no effect. A slight reduction in ATP levels was observed when the 3D-bioprinted human liver tissue model was further incubated without APAP for 3 and 18 h. Interestingly, 6 h of APAP treatment at 30 mM significantly reduced tissue glutathione (GSH) levels to 56% of the untreated control level (Fig. 3A), but had no effect on ATP levels (Fig. 3B). Moreover, tissue GSH levels gradually recovered after washout of APAP and additional incubation for 1, 3, and 18 h (Fig. 3A). This result shows the presence of homeostatic GSH synthesis in the 3D-bioprinted human liver tissue and thus suggests the biologically viable nature of the culture model. Taken together, these findings suggest that reconstituted human liver models such as 3D-bioprinted human liver may be useful for assessing hepatotoxicity after long-term repeated drug treatment and exploring the washout effects.

Tissue ATP levels after treatment with APAP for 1 (A), 7 (B), 14 (C), and 28 (D) days are plotted. Mean ± standard deriration (S.D.) The data were presented in The Society of Toxicology 55th Annual Meeting.24) (Color figure can be accessed in the online version.)
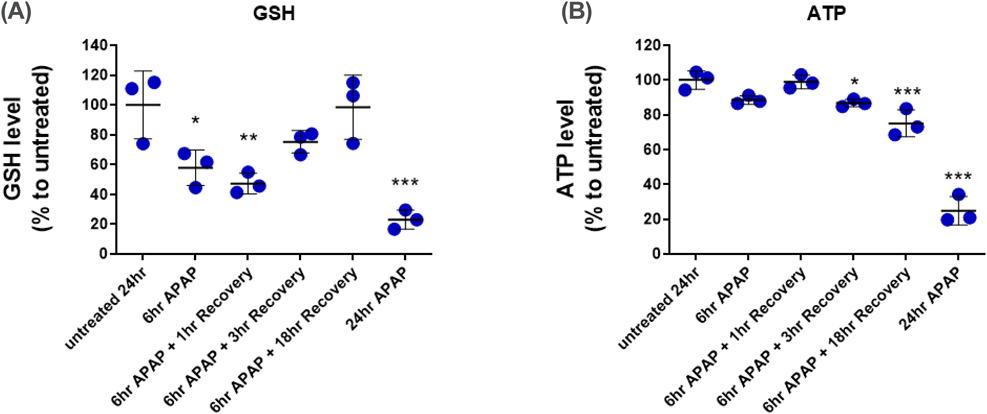
GSH levels decreased after 6 h of APAP (30 mM) treatment. Further culture after removal of APAP led to recovery of GSH levels to that of untreated controls. Reduction of ATP by APAP in the model was less severe when a recovery period was allowed. * p < 0.05, ** p < 0.01, and *** p < 0.001 by ANOVA (n = 3). The data were presented in The Society of Toxicology 55th Annual Meeting.24) (Color figure can be accessed in the online version.)
Given the increasing attention to and expectations of reconstituted human organ models, strategies to implement these models in a pharmaceutical company are becoming increasingly important. Pharmaceutical companies can be grouped as “users” of these models, while key players in their development such as academia and biotechnology companies, including start-ups, can be grouped as “developers.” Among the strategies that users can adopt to access these models, the first is the fee-for-service option. In this option, pharmaceutical companies can expect to benefit from developer-validated models. However, this type of service is only available after biotechnology companies with sufficient resources have established businesses to provide them, limiting opportunities for access to state-of-the-art models. In addition, this fee-for-service option is not applicable to test articles with logistical restrictions. Some biotechnology companies offer the fee-for-service option to potential customers based on various conditions listed on their website.25,26)
The second option is for the user to purchase human organ models from vendors and to conduct their own studies. A number of ready-for-use organ models are available.27–29) While the current lineup of ready-for-use organ models appears to be limited, pharmaceutical companies testing their propriety molecules are flexible in the study design used.
The third option is to implement “systems” such as organ-on-a-chip devices, pumps, and bioprinters. One advantage of this option may be that the user can establish a number of organ models from one specific platform. Users may be able to work with a variety of cell sources available in their stock. In fact, some biotechnology companies sell their developed devices.30–32) However, this option will require the users to maintain and troubleshoot the systems themselves. Some transfer of the technology’s processes, such as operation of equipment, construction of organ-on-a-chip components and their disassembly, and tips for cell culture using the devices, will also be needed. Recently, Sakolish et al.33) reported a case study on the transfer of technology of the human proximal tubule tissue chip from the original developer to their own laboratory. The researchers attempted to culture various types of human renal proximal tubular epithelial cells on a MPS platform provided by the original developer.34) By following the developer’s method for constructing the proximal tubule tissue chip, Sakolish et al. successfully cultured these cells in a 3D manner on the MPS platform. However, they were unable to reproduce some biological data from the original study. Sakolish et al. concluded that technology transfer of MPS is doable, although data reproducibility may depend on the cell source used in the MPS. This article is very informative for pharmaceutical companies considering successful technology transfer aimed at improving implementation of human organ models. Devices for constructing organ models can be successfully installed by pharmaceutical companies using the appropriate technology transfer process, although the user may be required to validate the organ model on a case-by-case basis. Additional case reports are expected in the future, which should in turn increase confidence in the distribution of this technology.
The final option is to undertake more interactive development of human organ models. In this option, organ models will primarily be established by biotechnology companies with the specialized knowledge, background, and skills. Pharmaceutical companies can work with the biotechnology companies by informing their requests and requirements for organ models, providing experimental ideas for evaluation of the organ models, and, in some cases, funding. Once an organ model prototype is established, pharmaceutical companies may request certain studies be conducted to evaluate the model. When finalized, the organ models will be used by biotechnology companies to assess test compounds. In addition, the technology and culture protocols will be transferred to the partnering pharmaceutical companies. In the past few years, there have been a number of press releases notifying a contract partnership between biotechnology and pharmaceutical companies.35–37) By choosing this option, users can immediately benefit from the latest advances in reconstituted human organ models. In addition, the organ models and associated cell sources will fit the specific aims of the pharmaceutical company. Achievement of such collaborative work is in part evident through publications co-authored by biotechnology and pharmaceutical companies. Table 2 summarizes selected co-authored publications on liver, kidney, intestine, and vascular tissue models by MPS developers and pharmaceutical companies. We propose that this type of interactive collaboration will most likely accelerate the development of improved organ models, and therefore result in benefits for both parties.
| Organ | Model developer | Pharmaceutical company | Reference |
|---|---|---|---|
| Liver | Organovo | Roche | 21) |
| InSphero | AstraZeneca and Genentech | 49) | |
| Emulate | AstraZeneca | 50) | |
| Kidney | Wyss Institute | Roche | 51) |
| MIMETAS | Astellas | 52) | |
| Small intestine | MIMETAS | Roche | 53) |
| Organovo | Merck | 54) | |
| Vascular including blood–brain barrier | AIST | Daiichi Sankyo | 55) |
| MIMETAS | Biogen | 56) | |
| Chiba Univ. | Eisai and Ono Pharmaceuticals | 57) |
Current uses of human organ models in a pharmaceutical company likely include the assessment of pharmacokinetics, safety, and efficacy of NMEs as described in this article and other reviews.38–40) Additionally, these organ models may also be useful for other drug discovery approaches such as phenotypic-based screening. Phenotypic screening is a method used to discover NMEs or lead molecules by focusing on substantial responses. In contrast, target-based screening focuses on interactions between molecules and specific targets such as receptors, ion channels, kinases, and other proteins. Swinney41) analyzed the strategies used in the discovery of drugs that were newly approved by the U.S. Food and Administration between 1999 and 2008, and reported that 37% of first-in-class drugs were identified using a phenotypic-based approach, a much higher rate than that for the target-based approach. This report suggests that improving the quality of models and increasing the variety of models may result in greater opportunities to identify first-in-class NMEs. Reconstituted human organ models that can be used to detect physiological organ responses may be a good choice for phenotypic screening in drug discovery. In particular, recent reconstituted human organ models have evolved to be more complex and more physiologically relevant as the trend in the development of these models has moved to establishing multiple-type cell models (co-culture models) and even organ-connected models (organs-on-a-chip or multiorgans-on-a-chip). These models can aid in the identification of new NMEs by enabling researchers to examine cell-to-cell interactions and organ-to-organ interactions, such as the effects of biological signals released from one organ to another organ modeled on a chip. For example, combining tumor organ models and immune cell incubation may lead to the discovery of new NMEs for cancer through immunomodulation.
Despite increasing interest in the use of reconstituted organ models for phenotypic screening, there are currently a number of challenges. One obstacle to phenotypic screening using reconstituted human organ models is obtaining sufficient throughput. For example, while the 384/1536-well format is popular in the first compound screening stage, to our knowledge, current MPS devices only allow preparation of a maximum of 96 organ chips in one device. This issue of throughput will likely arise once the usefulness of reconstituted human organ models in phenotypic screening is verified and efforts for miniaturization are accelerated. Another challenge is the robustness of the assay for screening. Sakolish et al.33) noted that the choice of cell source will affect the reproducibility of data among laboratories in the technology transfer process for MPS. They also noted that the use of renewable cells is an important factor in the development of reproducible models. In contrast, cells obtained from a primary cell source are generally expected to exhibit higher functionality, but may not be amenable to large-size screening approaches. We suggest the need to strike a balance between advantages and disadvantages when choosing a cell source. If the size of the compound library is small, reconstituted human organ models with primary cells may be sufficient for the phenotypic approach. In this regard, differentiated cells obtained from renewable stem cells would be an excellent option for use with reconstituted organ models for the screening approach. In addition to the choice of cell source, selection of the assay and readout are also key considerations for effective phenotypic screening. A simple and non-invasive assay is of practical importance in high throughput studies. While real-time monitoring, fluorescence-based assay, and serial measurement of transepithelial/endothelial electron resistance may be effective choices, it is also important to examine the correlation of these responses with their physiological significance. Regarding automation of processes such as plate preparation, cell culture, and assays, Kane et al.42) recently reported their efforts to combine their microfluidic culture devices with robotic systems. Peel et al.43) also reported their attempts to automate assays with organ-on-a-chip systems. They generated a bespoke adaptor that accepts two different organ-on-a-chip device designs for their imaging instruments so that the unique shapes of the organ-on-a-chip devices fit into their high-throughput-compatible instruments. Such automation efforts will improve ease of use of reconstituted human organ models for phenotypic screening.
Once the concept of human organ models has become more widespread and their utility has improved, the next step is to examine their utility for representing individual differences among humans. In fact, the current utility of reconstituted human organ models is fundamentally based on the premise that they can represent processes occurring in humans as a group, not as individuals. There are two potential ways that reconstituted organ models can be used to represent responses resulting from individual differences in humans. One is to develop reconstituted organ models from the cells of individuals, and to evaluate the individual differences in responses. Cell sources can be primary cells obtained from individuals including patients, or cells differentiated from the individuals’ stem cells. In this regard, quality control of the cells and the robustness of the system are very important; it is easy to see the potential difficulty of distinguishing between human-to-human differences, lot-to-lot differences of cell sources, and experimental deviation. Despite potential challenges, some studies have reported the successful determination of human-to-human differences. Emori et al.44) constructed and characterized 3D-cultures of fibroblast-like synoviocytes from rheumatoid arthritis (RA) patients and osteoarthritis (OA) patients. They showed that the 3D-RA and 3D-OA models differed in architecture and morphology. In addition, mRNA expression of proinflammatory mediators such as MMP1, MMP3, and TNFSF11 were higher in the 3D-RA model than the 3D-OA model. Interestingly, such differences between RA and OA were not observed in 2D-cultures. These results suggest that 3D-cultures can successfully depict disease-associated characteristics. In another example, Retting et al.45) compared the characteristics of 3D-bioprinted human liver tissue models prepared using healthy donor-derived cells with those prepared using diseased donor-derived cells, the latter of which was constructed using normal hepatocytes and diseased non-parenchymal cells from donors with confirmed non-alcoholic fatty liver disease/non-alcoholic steatohepatitis (NAFLD/NASH) and fibrosis. The diseased 3D-bioprinted human liver tissue model showed fibrotic profiles such as collagen deposition. Interestingly, this collagen deposition was observed in the absence of chemical stimuli such as transforming growth factor-beta, which is needed to induce fibrotic profiles in the “healthy” 3D-bioprinted human liver tissue model. These examples highlight the potential for reconstituted human organ models derived using diseased cell sources to depict the respective disease characteristics, enabling the discovery of patient-specific responses.
Alternatively, investigating differences among individuals using reconstituted human organ models may require a reversal in thinking. Namely, instead of developing human organ models from patient-derived cells, patient-derived specimens can be exposed to a human organ model. In this context, the human organ model may be considered a “human representative” or simply a diagnosing system. Very recently, Petrosyan et al.46) reported a newly developed human glomerular-on-a-chip model. They exposed the glomerular-on-a-chip to serum from patients with membranous nephropathy (MN), an autoimmune disease that induces the production of anti-podocyte antibodies and disrupts glomerular function,47,48) and investigated barrier function in the model. They observed changes in fluorescein-labeled albumin permeation in the glomerular-on-a-chip model. In addition, they observed a correlative trend between the extent of clinical proteinuria in MN patients and the extent of barrier dysfunction in the glomerular-on-a-chip model following exposure to serum from MN patients. This report suggests that reconstituted human organ models can be used to detect patient-dependent responses. Identifying the context in which a system can be used in drug discovery and development will be useful for personalized medicine. One option is to use the model for diagnosing patients’ disease stage. Additionally, the model may be useful for testing the effects of drugs in individual patients by co-treating patient-derived specimens with the drugs and evaluating their effect on dysfunction caused by patient-derived specimens.
Human organ models are an attractive tool in drug discovery as a complement to or substitute for animal models. Among human organ models, reconstituted human organ models are increasingly being used in life science research following recent progress in tissue engineering, micro-electro mechanical systems, and materials science. These models are useful for evaluating key physiological events in humans in a complementary manner to animal studies. They will also be useful for mechanistic investigation of events observed in human clinical trials. Further, they can be used to monitor biomarkers of organ-specific events in humans. In some cases, reconstituted human organ models have detected human responses that were not observed in corresponding animal studies. This emphasizes the value of reconstituted human organ models. We evaluated the utility of a 3D-bioprinted human liver tissue model and demonstrated the benefits for investigating the long-term effects of APAP on the liver, as well as the washout effect. This model proved useful for showing the chronic effects of hepatotoxicants and their detailed mechanisms. Such findings are expected to aid in the safety assessment of NMEs prior to clinical trials and to reduce unexpected failure of clinical trials due to hepatotoxicity. Further, the model can be used for mechanistic investigation of hepatotoxicity that arises in a clinical trial. While this review described a case using a liver model, the benefit of human organ models is also applicable to other organs. In addition, models that enable long-term culture while maintaining physiological functionality may allow researchers to investigate disease progression; such models are presumably currently being actively researched. Ultimately, assessment of the efficacy of NMEs will also be possible using human organ models. This is expected to be followed by their miniaturization and automation to increase throughput for application of this technology in phenotypic screening. To further leverage this momentum, current evidence suggests the importance of collaborative relationships between organ model developers and users for rapid installation of these models in the drug discovery process. It is our hope that the many forthcoming efforts to advance this technology and its applications will result in the delivery of new and improved medical solutions to patients.
The authors declare no conflict of interest.