2020 年 43 巻 4 号 p. 584-595
2020 年 43 巻 4 号 p. 584-595
The last few years have witnessed a great advance in the development of nonviral systems for in vivo targeted delivery of nucleic acids. Lipid nanoparticles (LNPs) are the most promising carriers for producing clinically approved products in the future. Compared with other systems used for nonviral gene delivery, LNPs provide several advantages including higher stability, low toxicity, and greater efficiency. Additionally, systems based on LNPs can be modified with ligands and devices for controlled biodistribution and internalization into specific cells. Efforts are ongoing to improve the efficiency of lipid-based gene vectors. These efforts depend on the appropriate design of nanocarriers as well as the development of new lipids with improved gene delivery ability. Several ionizable lipids have recently been developed and have shown dramatically improved efficiency. However, enhancing the ability of nanocarriers to target specific cells in the body remains the most difficult challenge. Systemically administered LNPs can access organs in which the capillaries are characterized by the presence of fenestrations, such as the liver and spleen. The liver has received the most attention to date, although targeted delivery to the spleen has recently emerged as a promising tool for modulating the immune system. In this review, we discuss recent advances in the use of LNPs for cell-specific targeted delivery of nucleic acids. We focus mainly on targeting liver hepatocytes and spleen immune cells as excellent targets for gene therapy. We also discuss the potential of endothelial cells as an alternate approach for targeting organs with a continuous endothelium.
Gene therapy is defined as the use of different nucleic acids to express, edit, or silence specific genes in cells for obtaining specific therapeutic effects. The first nucleic acid sequences used in gene therapy were in the form of plasmid DNA (pDNA). More recently, RNA-based drugs have been introduced such as small interfering RNA (siRNA) and mRNA. Despite the great promise of gene therapy as a tool for treating various currently incurable genetic as well as acquired diseases, it faces several difficulties such as the toxicity of viral vectors used in delivery of nucleic acids and the inefficiency of nonviral or synthetic vectors designed to replace the viral ones.1–3) The inefficiency of nonviral systems could be explained by the lack of efficient delivery systems that sufficiently protect nucleic acids and deliver them in sufficient doses to their target sites, usually inside cells.2,4) However, the last few years have witnessed a great advance in the development of nonviral systems for in vivo targeted delivery of genes to different organs, particularly the liver. This advance was translated into the approval of the first RNA interference (RNAi)-based drug, Patisiran (Onpattro), for the treatment of hereditary transthyretin-mediated amyloidosis (hATTR).5–7) Patisiran is a lipid-based formulation encapsulating siRNA and efficiently targets liver hepatocytes after intravenous administration to cause efficient and durable silencing of the abnormal form of the transthyretin gene. This breakthrough in the field of nonviral gene delivery received substantial interest worldwide and clearly showed the importance of lipid-based systems for developing more approved drugs in the future.
Several synthetic vectors are available for gene delivery. One strategy depends on using conjugates of different nucleic acids with different functional devices such as peptides, polymers, sugars, proteins, antibodies, or aptamers.8,9) Another strategy depends on encapsulating the nucleic acids in nanoparticles (NPs) of appropriate size. Conjugate systems are small in size and can easily be eliminated from the body through glomerular filtration in the kidney.10) Furthermore, the nucleic acids in the conjugates are not protected and must be chemically modified to resist degradation with nucleases in the circulation. NP systems, on the other hand, are large enough to bypass kidney elimination and can provide more protection of nucleic acids in the circulation. The NPs used for gene delivery can be broadly classified into polymeric and lipid nanoparticles (LNPs). The latter provide several advantages including higher stability, low toxicity, and greater efficiency.11–14) In addition, systems based on LNPs can be easily modified with other ligands and devices for controlled biodistribution and internalization into specific cells.
To increase the efficiency and decrease the side effects of lipid-based gene vectors, the systems must avoid rapid clearance and inactivation in the serum and must have the ability to target specific cells in the body.15,16) The fate of LNPs in the body after systemic administration varies depending on the lipids used and the design of the nanocarrier. Generally, systemically administered LNPs carrying nucleic acids can access organs in which the capillaries are characterized by the presence of pores or fenestrations such as the liver and spleen.17–19) Parenchymal cells in these two organs are thus considered accessible to LNPs, which make them particularly interesting candidate organs for gene therapy. The liver has received the most attention so far, and several lipid formulations are in different clinical trial stages.16) Recently, more attention has been given to LNPs-targeted delivery to the spleen as a promising tool for modulating the immune system for the treatment of various immune-related diseases, especially cancer immunotherapy.20) An interesting alternate approach is to develop LNPs targeting the endothelial cells lining the blood vessels. This approach is particularly important for drug delivery to organs with a continuous endothelium (i.e., without fenestrations) since LNPs in the circulation are in direct contact with endothelial cells and this avoids the extravasation step required to transfer NPs out of blood vessels.
In this review, we discuss the recent advances in the use of LNPs for cell-specific in vivo targeted delivery of different nucleic acids. First, we describe the various types of LNPs available to date and follow the revolution of lipid materials used from conventional cationic lipids to more efficient ionizable lipids. Second, we discuss the targeted delivery of LNPs to specific cells after systemic administration. We focus mainly on liver hepatocytes and spleen immune cells, as these two organs are the most promising for gene therapy due to their physiological characteristics that facilitate the delivery of nucleic acids to different parenchymal cells. We also discuss endothelial cells as an alternate approach for targeting organs with a continuous endothelium. We mainly focus on delivery to the lung endothelium since it is implicated in various pulmonary diseases.
Cationic lipids were among the first synthetic materials used for gene delivery.21,22) They are mainly used in the form of cationic liposomes that have the ability to strongly bind and condense negatively charged nucleic acids into stable NPs called lipoplexes (LPXs) (Fig. 1). LPXs are generally described as multilayered lipid structures in which the nucleic acid is sandwiched.23) The nitrogen/phosphate (N/P) ratio is usually adjusted so that the NPs formed usually have a net positive charge. A high N/P ratio is used to completely neutralize the nucleic acid and to avoid aggregation of NPs if they do not have a sufficient charge. Furthermore, an excess positive charge facilitates the binding of LPXs to the negatively charged cell membranes. A wide variety of cationic lipids could be synthesized but the most commonly used cationic lipids are N-[2,3-(dioleoyloxy)propyl]-N,N,N-trimethylammonium (DOTMA) and 1,2 dioleolyl-3-N,N,N-trimethylammonium-propane (DOTAP). The inclusion of neutral helper lipids like dioleoylphosphatidylethanolamine (DOPE) usually improves the efficiency of LPXs through enhancing the fusion with endosomal membranes, resulting in improvement of endosomal escape.24)
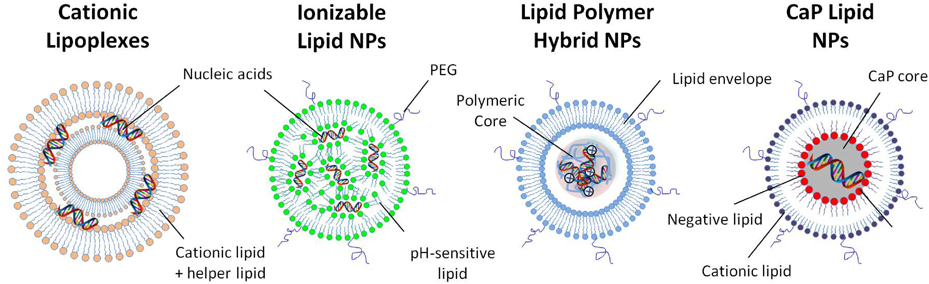
Cationic liposomes are composed of cationic lipids and neutral helper lipids and have the ability to strongly bind and condense the negatively charged nucleic acids into stable NPs called lipoplexes. Ionizable LNPs prepared with pH-sensitive lipids are suitable for systemic administration and avoid the disadvantages of LNPs with a permanent positive charge. Polyethylene glycol (PEG) is used for size control. Lipid polymer hybrid nanoparticles (LPHNPs) are composed of nucleic acids condensed into polymeric cores coated with lipid envelopes. Calcium phosphate (CaP) LNPs contain a CaP core coated with anionic lipids before coating with an outer lipid layer composed of cationic lipid and PEG. (Color figure can be accessed in the online version.)
Although cationic LPXs are very efficient in vitro, they are unstable in aqueous solutions and have limited efficiency in vivo due to their short half-life in the circulation. When intravenously injected, they bind to nonspecific negative proteins in the circulation and are rapidly cleared by organs of the reticuloendothelial system (RES).25,26) Furthermore, as the amount of cationic lipid used in LPXs is increased, the toxicity and immunogenicity of NPs increase.27) Nonspecific interactions with serum proteins can be decreased by coating LPXs with hydrophilic polymers like polyethylene glycol (PEG), resulting in long-term circulation of NPs. Although these long-circulating PEGylated LPXs have greater potential for accumulation in tumor tissues through the enhanced permeability and retention effect, they are not highly internalized into tumor cells and show limited transfection efficiency.28,29) Efforts are ongoing to improve the internalization into tumor cells using specific active targeting ligands that bind to specific cell surface receptors overexpressed in tumor cells.15) However, the actively targeted LPXs are still not sufficiently efficient.
2.2. Ionizable LNPsThe cationic charge of lipids is required for condensing nucleic acids, although it impairs the biodistribution of LNPs to specific tissues in vivo. Ionizable cationic lipids were introduced as a smart solution for this dilemma. These lipids have an apparent pKa value of <7 and thus they are cationic in acidic medium and neutral in physiologic conditions. They are used to interact with and condense nucleic acids under acidic (pH = 4) conditions, and then the medium is changed to pH = 7.4 so that the LNPs formed are neutral. These neutral LNPs are suitable for systemic administration and avoid the disadvantages of LNPs with a permanent positive charge.30)
LNPs prepared with ionizable or pH-sensitive cationic lipids have two more advantages. First, they bind to endogenous proteins in the circulation such as apolipoprotein E (ApoE), which efficiently mediates their delivery to hepatocytes via the low-density lipoprotein (LDL)-receptor pathway.31) Second, at acidic pH of the endosomes, these lipids acquire a positive charge and can bind to negatively charged lipids in the endosomal membranes forming nonbilayer structures that induce endosome disruption and result in improved endosomal escape. The use of ionizable lipids for siRNA delivery produces much greater gene silencing compared with cationic lipids in vitro owing to this efficient endosomal escape process.32) The LNPs formed between ionizable lipids and nucleic acids are generally described as stable nucleic acid lipid particles. As cryo-TEM images indicated, these NPs are composed of hydrophobic cores consisting of lipids encapsulating nucleic acids surrounded by other helper and PEG lipids to form stable NPs.33)
The early ionizable lipids used were 1,2-dioleoyl-3-dimethylammonium propane (DODAP) and 1,2-dioleyloxy-N,N-dimethyl-3-aminopropane (DODMA).34,35) However, the first ionizable lipid producing efficient gene silencing in hepatocytes after systemic administration was an ether analogue of DODAP called DLin-DMA.36) Other versions of ionizable lipids producing more efficient gene silencing are DLin-KC2-DMA (KC2) and DLin-MC3-DMA (MC3).37,38) The latter has an apparent pKa of 6.44 and showed the highest activity of siRNA delivery.38) YSK05 is another ionizable lipid, which showed high endosomal escape and high gene delivery to hepatocytes in vivo.32) YSK13-C3 is a more potent derivative of YSK05 with improved fusiogenic activity.39) The most efficient lipid in this series is CL4H6, which proved to be superior to MC3 lipid in the hepatic delivery of siRNA.40)
2.3. Lipid Polymer Hybrid NPsLipid polymer hybrid NPs (LPHNPs) are composed of nucleic acids condensed with both lipids and cationic polymers. The nucleic acids are first condensed with a polycation such as poly-L-lysine or protamine to form a core of positive or negative charge depending on the NP ratio used. This core is further coated with a lipid carrying the reverse charge to form stable NPs (Fig. 1). These systems combine the advantages of cationic lipids and polymers in gene delivery. The polycation commonly used is protamine, which protects nucleic acids during preparation and facilitates their encapsulation into LNPs. Furthermore, it decreases the amount of cationic lipids used and improves the nuclear delivery of DNA.41) The outer lipid layer provides more protection to nucleic acids and can be modified with different ligands and devices for improving pharmacokinetics and targeting of specific cells. One example of LPHNPs is the multifunctional envelope-type nanodevice (MEND) first described by Kogure et al.42) The MEND system was upgraded with various modifications to enhance the efficiency of gene delivery in vitro and in vivo.43–45) Remarkably, the use of octaatgrinine (R8) peptide significantly enhanced the cellular uptake and nuclear delivery, and the use of GALA peptide enhanced endosomal escape.46–48) The combination of R8 and GALA peptides produced very efficient MENDs based on synergism between the two peptides.49) Different MENDs were prepared encapsulating pDNA, siRNA, or mRNA and could be successfully used for efficient gene delivery in vitro and in vivo.50–53)
Recently, Khalil et al. have proposed a new 2-step coating strategy for a MEND system encapsulating pDNA and combining the R8 peptide with the ionizable lipid YSK05.54) This strategy showed significantly improved gene expression in HeLa cells compared with MENDs prepared with the conventional 1-step coating, owing to improved endosomal escape and nuclear delivery. Different versions of the MEND system were prepared for targeting different tissues in vivo including tumors, lung endothelium, and liver hepatocytes.55,56)
2.4. Lipid Calcium Phosphate NPsLipid calcium phosphate (LCP) NPs have recently been developed as a versatile platform for the delivery of various nucleic acids.57–59) The nucleic acids are condensed with calcium phosphate (CaP) to form a core that is then coated with different lipids to form core-shell structures called LCP-1. The CaP core is pH sensitive since the calcium and phosphate ions are released in the acidic medium of the endosomes and raise the osmotic pressure, resulting in the swelling of the endosome, and its bursting leads to improved endosomal escape. The CaP core in the next versions of LCP NPs was coated with anionic lipids such as dioleoylphosphatidic acid (DOPA) before coating with an outer lipid layer composed of the cationic lipid DOTAP, cholesterol, and PEG lipids60) (Fig. 1). The LCP NPs developed are characterized by a relatively small diameter of <50 nm, which facilitates delivery to hepatocytes through liver fenestrations. Different versions of LCP NPs were successfully used for delivering various nucleic acids in vivo.59) In the case of pDNA delivery, the presence of the R8 peptide in the DNA core significantly enhanced nuclear delivery, resulting in high gene expression in hepatocytes.60)
Delivery of nucleic acids to the liver has received substantial interest. This can be attributed to the importance of the liver as a multifunctional organ in the body which can be affected by various diseases, including inflammation, fatty liver, viral hepatitis, cirrhosis, and hepatocellular carcinoma (HCC) as well as a wide variety of metabolic and genetically inherited diseases.61–64) According to recent surveys, liver diseases account for 2 million deaths annually.65) Most liver diseases are considered incurable with only palliative treatment available, while the others have limited therapeutic approaches. Gene therapy has resuscitated the hope of providing effective treatments for these diseases.66)
LNPs are particularly useful for gene therapy applications in liver diseases due to the relative ease of targeting hepatocytes (Fig. 2). First, the liver is a well-perfused organ, which makes it accessible to NPs circulating in the blood. Second, the liver endothelium is discontinuous and contains fenestrae of roughly 100–150 nm which exhibit high extravasation of macromolecules out of the circulation to liver hepatocytes.67) Third, the endogenous lipid uptake mechanism facilitates the uptake of lipid systems from the circulation to hepatocytes. Despite these features that promote targeting to hepatocytes, the delivery of genetic payloads to the liver is challenged by the presence of macrophagocytic Kupffer cells (KCs) that can engulf the injected cargos before reaching the cells of interest in the liver.68) Thus, the appropriate design of delivery systems that can escape this pathway and be internalized into the target cells is critical.
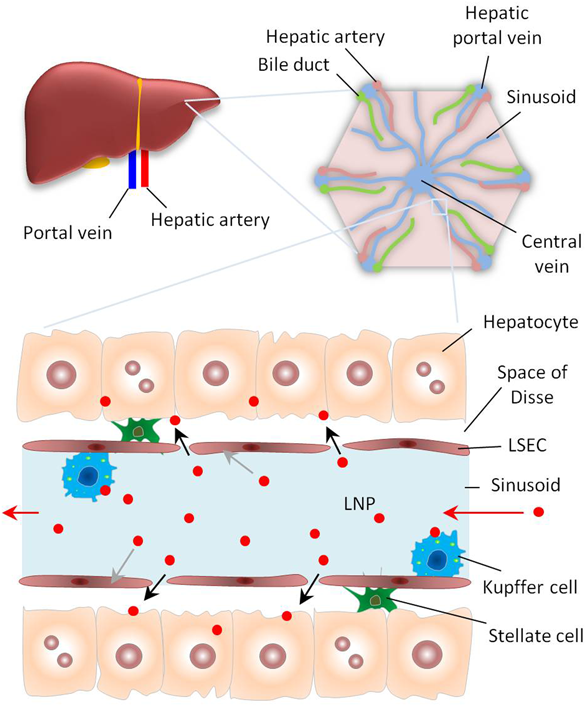
The liver has a dual blood supply from the portal vein (75%) and hepatic artery (25%). In a classical liver lobule, the blood drains from the hepatic portal veins and hepatic arteries into the central veins in liver capillaries called sinusoids. These sinusoids have increased permeability since the liver sinusoidal endothelial cells (LSECs) have gaps, which allow LNPs to enter and leave the bloodstream and be delivered to hepatocytes. The liver macrophages (Kuppfer cells) in the sinusoids can take up and destroy foreign materials including LNPs. Hepatic stellate cells (HSCs) involved in liver fibrosis are present in the space of Disse. (Color figure can be accessed in the online version.)
Cationic liposomes were the first generation of LNPs used for nucleic acid delivery to the liver. They can be simply classified into two categories: liposomes prepared with cationic lipids (e.g., DOTAP); and liposomes modified with cationic peptides. In either case, negatively charged nucleic acids are complexed by positively charged lipids or peptides to form LPXs with a final positive charge.69,70) Mohr et al. investigated cationic liposomes based on the cationic lipid GL-67 to deliver pDNA encoding the chloramphenicol acetyltransferase (CAT) reporter gene to various organs, and the system showed superior gene delivery to the liver over other organs. It was then applied to gene delivery to intrahepatic HCC tumors established in nude mice.71) Khalil et al. developed pDNA-containing LNPs prepared mainly with the neutral lipid DOPE.51) The pDNA is first condensed with the polycation polyethyleneimine (PEI) to form cores that are then coated with a lipid envelope modified with the cationic stearylated-R8 (STR-R8) and cholesterol-GALA peptides. The R8 peptide shows a high affinity for hepatocytes, while the GALA peptide functions as an endosomal escape device. This system could efficiently deliver several nucleic acids to the liver including pDNA, siRNA, and oligonucleotides.51,53,72)
Surprisingly, the use of negative PEI/DNA cores was significantly superior to the use of positive cores, probably due to the ease of release of DNA from negative less-condensed cores.51) Huang and colleagues developed a specific LCP system for pDNA delivery to hepatocytes after intravenous injection.60) The pDNA was complexed into a core based on CaP- and arginine-rich peptides, which is further coated with negative lipid DOPA followed by the cationic lipid DOTAP. This system showed highly efficient gene transfer in the liver. The high efficiency was attributed to the powerful endosomal escape of the CaP/DNA core and improved nuclear delivery mediated by the arginine-rich peptide.
3.2. Ionizable Lipids for Hepatic Gene DeliveryDespite their high efficiency, cationic liposomes have several drawbacks as explained above. Therefore, the second generation of LNPs was based on pH-sensitive cationic lipids. As explained earlier, these lipids overcome the common demerits of lipids with permanent cationic charges. In addition, these lipids can better benefit from the endogenous serum carrier ApoE, which directs NPs to hepatocytes where they are taken up through LDL receptors.31) Zimmermann et al. reported the first efficient gene silencing in hepatocytes using LNPs encapsulating siRNA and prepared with the ionizable cationic lipid DLin-DMA.36) These LNPs could successfully silence the apolipoprotein B (ApoB) gene in the hepatocytes of cynomolgus monkeys, an example of nonhuman primates. Semple et al. introduced a more efficient lipid, KC2, which was successfully used for efficient silencing of the coagulation factor VII (FVII) gene in hepatocytes (the ED50 value was 0.01 mg/kg).37) Increasing the diameter of KC2-based LNPs from approx. 100 to approx. 300 nm significantly decreased liver delivery and enhanced delivery to macrophages and dendritic cells (DCs) in the bone marrow.73)
A screening study based on derivatives of the KC2 lipid with pKa values ranging from 4.17 to 8.12 was used to select a more efficient variant, MC3, which had a pKa value of 6.44.38) MC3-based LNPs produced dramatically efficient silencing of the FVII gene in hepatocytes (with a IC50 value of 0.005 mg/kg). MC3 was the main lipid component in LNPs that successfully delivered siRNA to hepatocytes to treat hATTR which was approved by the U.S. Food and Drug Administration (FDA) as the world’s first RNAi therapy marketed under the name Patisiran.
The high efficiency of gene silencing obtained with these LNPs triggered a rapid competition among scientists to develop new generations of these smart lipids. Sato et al. developed the pH-sensitive cationic lipid YSK05, which efficiently delivered siRNA against FVII to hepatocytes with higher gene knockdown efficiency than the commercially available pH-sensitive lipid, DODAP.32) YSK05 LNPs loaded with siRNA against hepatitis C virus (HCV) inhibited HCV in mice with persistent infection.74) A derivative with higher fusiogenic ability, YSK13-C3, was more potent than YSK05 as the main lipid for siRNA delivery.39) YSK13-C3-LNPs loaded with siRNA against hepatitis B virus (HBV) produced a strong and durable inhibition of HBV antigens and DNA.75) Recently, a library of different ionizable lipids having variable lipid, linker, and amine heads has been prepared and screened for efficient gene silencing in hepatocytes. The optimal lipid for gene silencing in hepatocytes was found to be CL4H6 with an IC50 value of 0.0025 mg/kg, which is half the IC50 value of MC3-based LNPs.
An extensive investigation of the structure–activity relationships of these lipids was conducted to understand the criteria controlling their activity and consequently to rationalize their design to maximize efficiency. The optimum particle size to target hepatocytes was 76.5–117 nm, while increasing particle size up to 200 nm increased the selectivity toward liver sinusoidal endothelial cells (LSECs).39) The intrahepatic distribution can also be controlled by the proper control of the pKa value and ionization status of LNPs. In a recent study, two lipids, YSK05 and YSK12-C4 (pKa values of 6.50 and 8.00, respectively), were mixed in different ratios to produce LNPs with different pKa values. LNPs with a pKa value of 7.15 and an appropriate ionization status (approx. 36% cationic charge) could successfully deliver siRNA to LSECs. On the other hand, LNPs with a pKa value of 6.50 showed maximum gene silencing in hepatocytes.76)
One important issue to be considered is the toxicity of LNPs based on ionizable lipids used for gene delivery to hepatocytes. The intravenous injection of these LNPs produces various pro-inflammatory cytokines. Therefore, several methods were examined to decrease the toxicity of these LNPs. One strategy is based on using biodegradable derivatives of commonly used lipids. A biodegradable version of MC3, called L319, was less efficient in siRNA delivery (IC50 value of 0.01 mg/kg) but showed an improved safety profile compared with the nonbiodegradable derivative.77) Similarly, the biodegradability of the CL4H6 lipid increased its tolerability and biosafety compared with the previous generations.40)
A second strategy is based on decreasing the amount of ionizable lipids. This was generally associated with decreased efficiency. The use of protamine for neutralizing the siRNA was used to restore the high efficiency of LNPs prepared with low amounts of ionizable lipids.78) It was reported that the liver toxicity of injected LNPs is related to the accumulation in LSECs, resulting in an elevation of inflammatory cytokines.79) Therefore, a third strategy to decrease hepatotoxicity was based on decreasing the delivery to LSECs using ligands for active targeting to hepatocytes. LNPs modified with N-acetyl-D-galactosamine (GalNAc) showed improved hepatocyte delivery and reduced toxicity compared with bare LNPs.79)
3.3. Active Targeting for Hepatic Gene DeliveryTo ensure selective internalization into hepatocytes, LNPs can be functionalized with various targeting ligands that have particular affinity for overexpressed receptors in the target cells. For example, galactose80) and GalNAc81) have been extensively used to target the asialoglycoprotein receptor in hepatocytes. Modification of LNPs prepared with KC2 lipid with the GalNAc ligand significantly improved siRNA delivery in ApoE-deficient mice. GalNAc has been also used for preparing a smart dynamic polyconjugate system.82) This system uses acid-labile PEG lipids that dissociate in the acidic medium of the endosome to facilitate endosomal destabilization. Tros de Ilarduya et al. used transferrin-modified LPXs for efficient delivery of luciferase-expressing pDNA to hepatocytes.83) The incorporation of protamine in LNPs significantly improved gene transfer efficiency, which was attributed to improved nuclear delivery.83) LNPs can also be targeted to liver cells other than hepatocytes. LNPs based on hyaluronic acid-modified sphingosine 1-phophate (HA-S1P) were developed to target HA receptors in LSECs to treat ischemia-reperfusion injury.84) Narmada et al. used vitamin A-coupled liposomes to deliver pDNA encoding the hepatocyte growth factor gene to activated hepatic stellate cells (aHSCs) for the amelioration of liver fibrosis.85) Huang’s group designed LCP NPs that were surface modified with aminoethyl anisamide to target the sigma-1 receptor in aHSCs and deliver pDNA expressing the antifibrotic peptide relaxin.86)
3.4. Next-Generation LNPs for Hepatic Gene DeliveryAlthough active targeting by specific ligands is widely used, challenges include the complexity of delivery systems, the probability of detachment of ligands from the nanocarriers after systemic administration, and the necessity for perfect ligand–receptor interaction.87) Therefore, attention has been directed toward the synthesis of biomaterials that allow endogenous transport proteins in the serum to be specifically accumulated in the desired organ.88) In addition, the chemical structures of these novel materials can be tailored to control their efficiency and selectivity or to impart desirable properties (e.g., biodegradability) to improve their biosafety.
Anderson’s group synthesized a combinatorial library of 1200 lipid-like materials (lipoids) and screened their ability to deliver siRNA or antisense oligonucleotides to various tissues in mice, rats, and nonhuman primates.89) Lipoid 98N12 resulted in more than 90% knockdown of the FVII gene in mice hepatocytes after intravenous administration of an siRNA dose of 2.5 mg/kg.89) In a second study, a combinatorial epoxide-derived lipidoid library was synthesized and screened for in vivo potential. LNPs based on C12-200 lipoid showed specific simultaneous knockdown of five hepatic genes, FVII, ApoB, PCSK9, Xbp1, and SORT1, after a single intravenous injection in mice at an siRNA dose of 0.2 mg/kg for each siRNA. Furthermore, highly efficient gene silencing of the transthyretin gene was achieved in the livers of nonhuman primates at an siRNA dose of 0.03 mg/kg.90)
Cullis and colleagues synthesized a library of biodegradable lipids for efficient siRNA delivery to hepatocytes.91) The biodegradability of those novel lipids enhanced biotolerability and facilitated rapid elimination of LNPs after exertion of their role.91) Sato et al. also used the same concept to develop their efficient and tolerable CL4H6 lipid for hepatocyte gene delivery.40)
The spleen is the largest lymphatic organ in the body and contains different types of immune cells such as T cells, B cells, DCs, and macrophages. The spleen is characterized by the presence of both open and closed circulation pathways20) (Fig. 3). In the open circulation pathway, the arteries deliver the blood directly to the parenchymal cells. In the closed circulation pathway, the arteries deliver the blood to the parenchymal cells via splenic sinusoids with pores ranging from 200 to 500 nm in size. In addition, the blood flow in the spleen is slow compared with that in other organs. These features make the spleen a relatively accessible organ to intravenously injected LNPs.17) Therefore, the spleen is a good candidate for gene therapy for the treatment of immune-related diseases including allergies, inflammations, infections, and cancer.20,92–94) The most critical point is to target specific types of spleen cells for achieving a level of gene delivery sufficient for producing a therapeutic effect. This section focuses on in vivo spleen-targeted gene delivery using lipid-based nanocarriers focusing mainly on delivering genes to specific spleen cells.
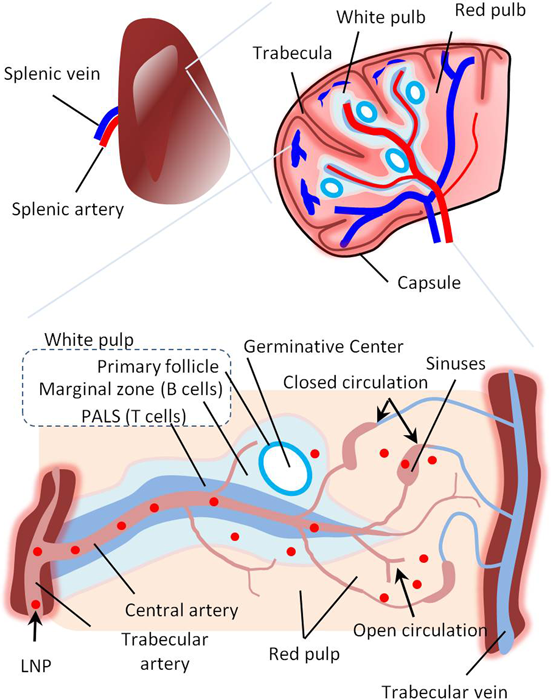
The spleen is coated with an outer capsule with trabeculae, which subdivide the spleen into lobules. The blood is supplied via splenic arteries and exits through splenic veins. The spleen consists of red and white pulp. The white pulp consists of lymphocytes that surround the spleen arteries (B lymphocytes in the marginal zone and T lymphocytes in the periarterial lymphatic sheath [PALS]). The red pulp consists of splenic sinuses and pulp cords composed of connective tissues containing red and white blood cells (including dendritic cells [DCs] and macrophages). The spleen has unique blood flow characterized by closed and open circulation. In the closed circulation, the capillary opens into the splenic sinuses, from which the blood empties into the splenic vein. In the open circulation, the capillary opens directly into the pulp cord and filters into the splenic sinuses. LNPs can pass the fenestrations in the marginal zone and be delivered to B lymphocytes. Otherwise, they will be filtered in the red pulp and delivered to DCs and macrophages. (Color figure can be accessed in the online version.)
As mentioned above, NPs administered intravenously are opsonized and distributed into RES organs, mainly the liver and spleen. The liver is the main organ receiving injected NPs (approx. 80%). Thus, liver uptake is considered a major hurdle for spleen targeting. Liver KCs are known as dominant phagocytic cells taking up intravenously injected NPs.95) It was reported that the depletion of KCs increased the spleen accumulation of NPs.96,97) Therefore, the avoidance of liver uptake is the most critical parameter when designing efficient spleen-targeted systems. Generally, different strategies can be used for avoiding hepatic uptake and enhancing spleen targeting.98,99) Adding PEG coats to LNPs is a known strategy for decreasing uptake by KCs. In addition, nonspherical NPs can also escape from KCs. Positive LNPs are more prone to liver uptake compared with neutral or negative ones. Size also affects spleen delivery, and large NPs (>200 nm) showed a higher concentration in the spleen compared with smaller liposomes (<200 nm). The flexibility or rigidity of NPs is also a key factor for spleen delivery.
The spleen has a function in removing old red blood cells (RBCs) via splenic filtration. Normal RBCs can pass through the slit of the spleen sinusoids, while old ones become trapped due to their rigidity. Through the same mechanism, more rigid NPs are thought to be trapped in the spleen. The inclusion of cholesterol in the liposomes, which increases the rigidity of the lipid bilayer,100) enhanced splenic uptake.101) Thus, the lipid composition of LNPs is important for enhancing spleen accumulation of lipid-based carriers.
4.2. Strategies for Spleen Cell TargetingUnlike hepatocyte-targeted delivery, the use of specific ligands for targeting specific cells in the spleen is not common. However, some lipids can be used for targeted spleen delivery. Spleen macrophages and DCs recognize phosphatidylserine, which is exposed on the outer surface of apoptotic cells as well as aging RBCs. Kurosaki et al. coated pDNA condensed NPs with an analogue of phosphatidylserine, 1,2-dioleoyl-sn-glycero-3-phospho-L-serine (DOPS), for enhancing spleen delivery.102) DOPS-PEI NPs were negatively charged and produced relatively high and selective gene expression in the spleen. Kranz et al. showed that delivery to spleen DCs can be achieved by LNPs formed from commonly used lipids, DOTMA and DOPE, by simply adjusting the charge ratio (lipid : RNA ratio) without using a specific ligand.103) Positive RNA-LPXs showed higher gene delivery in the lung compared with the spleen. Interestingly, a gradual decrease in the cationic lipid content shifted gene expression from the lungs to the spleen. LPXs carrying a high negative charge did not show efficient gene expression in any organ. Although the mechanism of spleen targeting was not clarified, the generally accepted hypothesis is that these slightly negative NPs bind to endogenous proteins, which mediates the efficient delivery to spleen cells. Recently, the role of the protein corona in organ targeting has attracted considerable interest.104–106)
In gene therapy, however, delivering therapeutic nucleic acids to target organs alone is not sufficient to yield an effective therapeutic outcome. Intracellular events responsible for efficient gene expression or silencing must be considered. Endosomal escape is a major barrier for nucleic acid-based therapeutics, and nuclear delivery is also necessary in case of DNA-based therapeutics. OF-Deg-Lin, an ionizable lipid with high endosomal escape ability, was designed by Fenton et al.107) LNPs encapsulating mRNA and prepared with OF-Deg-Lin exhibited spleen-selective protein production, although the LNPs were transported predominantly to the liver.107) While the mechanism by which LNPs are expressed predominantly in the spleen is unclear, the authors hypothesized that the electrophilic ester bonds in OF-Deg-Lin may degrade more readily in the liver compared with other organs. This hypothesis was supported by testing the gene expression of LNPs prepared with a nondegradable derivative, OF-02. Nondegradable LNPs produced more gene expression in the liver than in the spleen. This indicates that the difference in the stability of lipid formulations can be an efficient strategy for spleen-selective gene expression.
Gene silencing in specific spleen immune cells could be achieved using various LNPs containing siRNA.73,108,109) Kranz et al. demonstrated that increasing LNP size can improve antigen-presenting cell (APC)-specific silencing.103) One possible reason for this phenomenon is that the spleen accumulation of LNPs is enhanced by increasing their size, as mentioned above. Another possible reason is related to the internalization pathway into cells. It was reported that the uptake pathway affects transfection efficiency, and the particle size is one of the factors of determining the pathway.110) Sago et al. also discussed the internalization pathway as a factor affecting functional siRNA delivery.111) Both tissue distribution and intracellular distribution/trafficking should be considered for efficient cell type-selective transfection.
4.3. Applications for Spleen Gene DeliverySpleen-targeted gene delivery can be applied for stimulating efficient specific immune activation. Thus, studies on its applications to cancer immunotherapy and vaccines are in progress.103,112) An optimized RNA-LPX showed high spleen-selective gene expression, and antigen-encoding RNA-LPX mediated the rejection of progressive tumors. In that report, the authors compared systemic intravenous vaccine delivery with local subcutaneous delivery. The results showed that systemic delivery is more efficient with higher therapeutic potential than local delivery. This was explained based on a higher absolute number of transfected APCs. The RNA-LPX showed a high tumor rejection rate despite the low percentage of transfection to APCs in the spleen (approx. 5%). In addition to mRNA delivery, pDNA delivery is also a promising choice for cancer vaccines.113) Both antigen presentation and immune cell activation are important for efficient cancer immunotherapy. The pDNA-encoding tumor antigen has the potential to induce both antigen presentation and activation of immune cells because pDNA can stimulate APCs via Toll-like receptor 9 and/or other cytosolic DNA sensors.114,115) The use of pDNA encapsulating LNPs as an antitumor adjuvant was previously demonstrated.116)
Recently, there has been considerable interest in targeting B lymphocytes (or B cells) in the spleen. B cells not only produce antibodies and cytokines for fighting infections and inflammation, they can act as APCs for stimulating specific T cell production. Fenton et al. used an ionizable lipid for delivering mRNA to B cells.107) Rossetti et al. demonstrated that the activation of B cells to act as APCs could promote the antitumor effect.117) Colluru et al. showed that murine B cells, but not DCs, co-cultured with pDNA efficiently express the antigen and elicit antigen-specific T cell activity in vivo.118) Shen et al. reported that dextran NPs targeted B cells in the spleen through complement activation and produced strong antigen-specific activation.119) These NPs were used for preventing the induction of anaphylactic shock and allergic asthma. We showed that double-coated R8-YSK-MEND encapsulating an antigen pDNA targeted B cells in the spleen and could be successfully used as an antitumor vaccine.120) Those reports pointed out the importance of B cells as a possible target for gene therapy for different diseases.
In summary, spleen-targeted gene delivery has great promise for immunotherapy, especially cancer vaccination. It is likely to find clinical applications next to the liver-targeted carriers (e.g., Patisiran) due to its relative ease of targeting.
The endothelial cells lining all the blood vessels in the body are in direct contact with the blood in the circulatory system. Unlike the capillary endothelium in the liver and spleen, which contains relatively large pores or fenestrae, the endothelium in most organs of the body is continuous, with no large pores or fenestrae18) (Fig. 4). In organs with a continuous endothelium, LNPs must pass through the endothelial cells to reach other types of cells. This transendothelial delivery of NPs occurs mainly through transcytosis and is generally described as an inefficient process. However, the endothelium itself affects several physiological processes in the body such as metabolism, inflammation, and angiogenesis. It is implicated in diseases including cardiovascular and cerebrovascular ischemia, inflammation, diabetes, and tumor growth and metastasis. Delivering drugs and therapeutic genes directly to the endothelium is thus a promising tool for disease interference without the need for the extravasation of nanocarriers from the blood vessels.
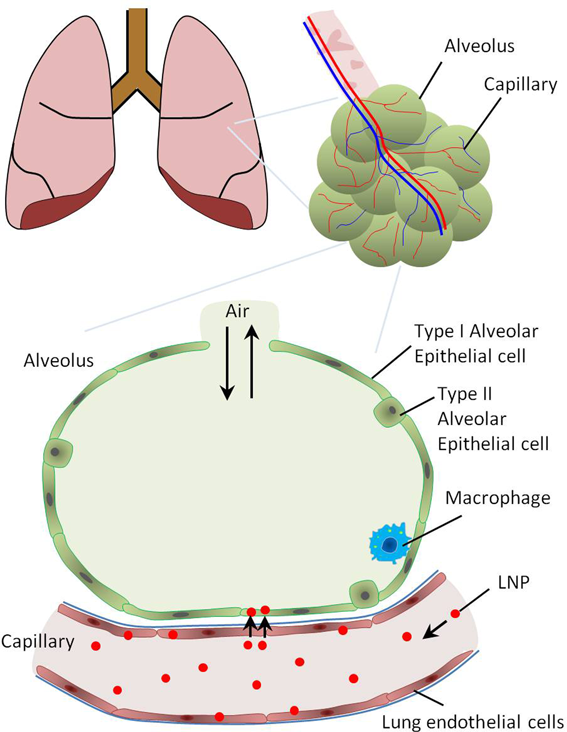
The respiratory portion ends with alveoli surrounded by blood capillaries. The epithelium of the alveoli contains type I and type II pneumocytes or alveolar epithelial cells. Alveolar macrophages are abundant and play important roles in lung immunity. The lung capillaries are lined with continuous lung endothelial cells, with no gaps or fenestrations. Intravenously injected LNPs passing the lung capillaries can be delivered to the lung endothelial cells. However, a fraction of injected LNPs may pass the capillaries and reach alveolar epithelial cells, mainly through transcytosis. This is facilitated by the presence of a very thin basement membrane between alveolar and endothelial cells. (Color figure can be accessed in the online version.)
Endothelial targeting is particularly effective for tumor therapy through the inhibition of angiogenesis. Tumor endothelial cells (TECs) are genetically more stable than tumor cells (TCs) and this decreases the resistance to chemotherapeutic drugs.19) In addition, TECs represent only a small fraction of the tumor, which decreases the dose of drugs needed compared with delivery to TCs. Unfortunately, there is no specific affinity of drugs and NPs for endothelial cells. Targeted NPs must be modified with specific antibodies and ligands that target specific adhesion molecules on the surface of endothelial cells. In this section, we discuss some recent reports showing successful targeting of the pulmonary endothelium as one of the tissues in the body most commonly implicated in diseases.
5.1. Gene Delivery to the Lung EndotheliumThe lung endothelium is a good candidate for gene therapy because it is implicated in different lung diseases, such as pulmonary hypertension and lung cancer.121) The lung endothelium is the first capillary encountered by intravenously injected NPs. Cationic LPXs usually aggregate in the circulation due to electrostatic binding with negative serum proteins, and these aggregates are generally entrapped in the lung capillaries.122) The aggregates are not internalized into lung endothelial cells (LECs) and gradually dissociate from the lung capillaries to enter the general circulation. However, some LPXs could be delivered successfully to LECs.
Atu027 is an LPX formed between siRNA against protein kinase N3 (PKN3) and cationic liposomes formed from cationic, helper, and PEG lipids.123,124) Atu027 caused significant PKN3 gene silencing in the pulmonary endothelium and reduced tumor growth in orthotopic mice models. Although it is currently in the clinical trial phase, the dose administered is large, and a more specific system would be useful. Dahlman et al. identified a low molecular-weight lipid polymer system, called 7C1, that encapsulates siRNA and showed strong and durable gene silencing in endothelial cells, particularly in LECs (the ED50 value was 0.02 mg siRNA/kg, and gene silencing continued at >75% for 21 d).125) 7C1 did not cause significant gene silencing in hepatocytes and immune cells in the lung but caused significant silencing in the endothelium of different organs such as the kidneys and heart. Therefore, a more specific system is definitely required for next-generation nanomedicine directed to the lung endothelium.
5.2. The GALA Peptide for Gene Delivery to Pulmonary CellsKusumoto et al. reported on the surprising ability of the GALA peptide to target the lung endothelium.56) Intravenous injection of GALA-modified liposomes (GALA-LPs) resulted in rapid accumulation in the lung compared with other organs. The liposomes did not aggregate and remained in the lung for a long duration. GALA-modified LNPs prepared with DOTMA and EPC and encapsulating antiCD31 siRNA were highly internalized into LECs in vivo and caused strong, selective CD31 gene silencing in the lung endothelium (ED50 value of 0.4 mg siRNA/kg). The system was used for controlling lung metastasis in a mouse tumor model. Attaching the GALA peptide to the tip of PEG spacers (DSPE-PEG-GALA) improved the activity compared with LNPs prepared with direct attachment of the GALA peptide to the lipid surface (cholesterol-GALA).126) The activity was further improved by using the ethanol dilution method of preparation and by adding a vitamin E-scaffold SS-cleavable pH-activated lipid-like material (ssPalmE), which improved the endosomal escape of siRNA (ED50 value = 0.21 mg siRNA/kg).127) Further improvement in the endosomal escape through using an ionizable lipid, YSK05, significantly improved the gene silencing activity in the lung endothelium (ED50 value = 0.01 mg siRNA/kg).50)
Although the use of YSK05 dramatically improved the activity by approx. 40-fold, the GALA/YSK05 system produced significant gene silencing in the liver endothelium. Surprisingly, modulation of the lipid composition by adding different helper lipids and changing the ratio of other components like cholesterol and PEG lipid significantly decreased liver gene silencing and restored lung selectivity. The optimum system was composed of YSK05, DOPE, cholesterol, and DMG-PEG, where the presence of the helper lipid DOPE was critical for decreasing the liver and enhancing the lung delivery. This interesting result shows that the targeting ability of systems prepared with active ligands can be improved by the optimization of other lipid components, particularly of helper lipids.
Recently, using confocal microscopy and FACS analysis, Santiwarangkool et al. have shown that GALA-modified LNPs delivered extensively to LECs can pass through the endothelium and reach other pulmonary cells including type I and type II alveolar epithelium and lung macrophages through transcytosis.128) This was confirmed by the ability of GALA-LNPs encapsulating antipodoplanin siRNA to knock down the podoplanin gene in pulmonary epithelial cells. Using electron microscopy, GALA-LNPs encapsulating gold nanoparticles were detected in LECs as well as in the basement membrane and other pulmonary cells. In contrast to the expectation that transcytosis is mainly linked to uptake through caveolae, GALA-LNPs were internalized via clathrin-mediated endocytosis. These results collectively showed that GALA-LNPs can be used as a vector for delivering genetic drugs not only to the lung endothelium but also to other pulmonary cells, which makes these LNPs an interesting platform for the treatment of various diseases associated with the lung epithelium. Lung delivery of pDNA was also achieved using LNPs modified with the short IRQ peptide.129,130)
LNPs are the most efficient nonviral systems for the delivery of nucleic acids in vivo. Some lipid-based products are approved for clinical use, and several candidates are in different clinical trial stages. Improving the activity of different lipid-based gene vectors depends on the appropriate design of lipid nanocarriers as well as the development of new lipids with improved gene delivery ability. However, enhancing the ability of lipid nanocarriers to target specific cells in the body after systemic administration remains the most difficult challenge. New molecular targets and targeting ligands must be identified and innovative nanotechnology must be developed to avoid the various complex extra- and intracellular barriers to gene delivery.
This work was supported by the Special Education and Research Expenses from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan.
The authors declare no conflict of interest.